1,2 and 1,4- Addition To Dienes
Description: Conjugated dienes treated with strong acids (e.g. HCl, HBr) may result in either “1,2-addition” products (top) or “1,4-addition” products, depending on temperature.
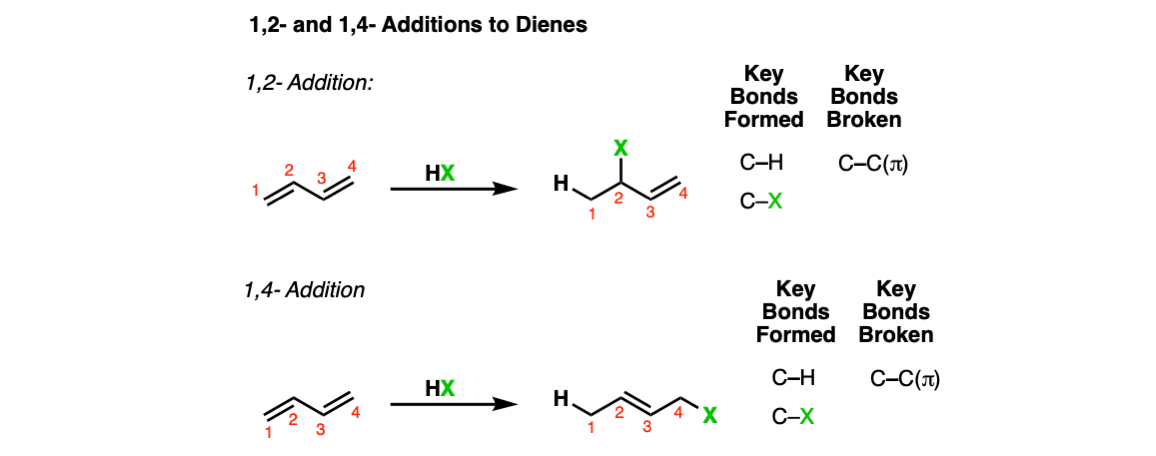
Notes: “HX” here is often HCl or HBr.
Examples:
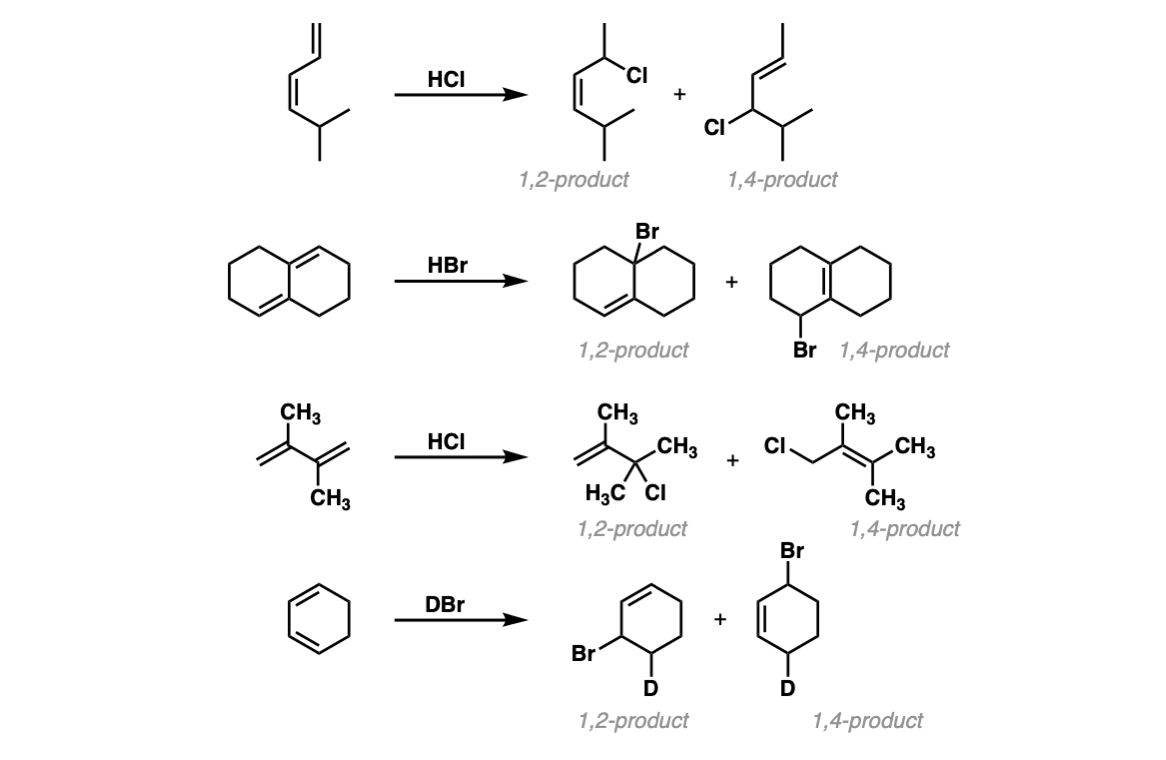
Notes:
The key factor in whether the 1,2- product or the 1,4-product is favored depends on temperature. Low temperatures will favor the product in which the most stable carbocation is favored (usually the 1,2-) and higher temperatures will favor the product which has the most substituted alkene (often the 1,4-).
See: Additions to dienes: 1,2- vs 1,4- addition
The first example gives two disubstituted alkenes, which should be about equal in stability. The second example has a 1,2-product with a trisubstituted alkene and a 1,4-product with a tetrasubstituted alkene, so the 1,4- should be favored under thermodynamic conditions. The third example has a disubstituted alkene (1,2) vs. tetrasubstituted alkene (1,4). The fourth example uses DBr instead of HBr, resulting in deuterated products (recall that D is the heavy isotope of hydrogen). In this case there isn’t a significant difference between the stability of the two products.
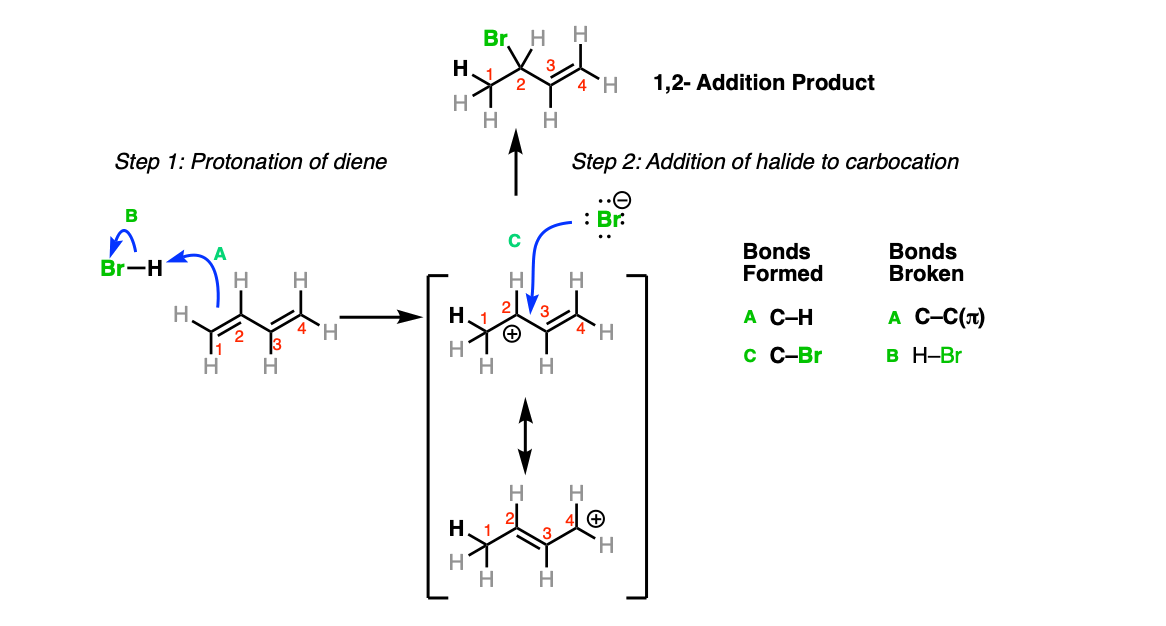
Mechanism: The “1,2-addition” product simply results from the protonation of the alkene in order to form a new, resonance-stabilized carbocation (Step 1, arrows A and B) followed by attack of the carbocation by the anion at the position adjacent to the carbon which was protonated (Step 2, arrows C and D).
Here’s the mechanism for the 1,4 product. The alkene is protonated by H-Br (Step 1, arrows A and B) resulting in a new resonance-stabilized carbocation. In this case, the bromide anion attacks not at the position adjacent to the new C-H (carbon 2) but instead carbon 4, which you can imagine as the resonance form below (Step 2, arrows C and D).
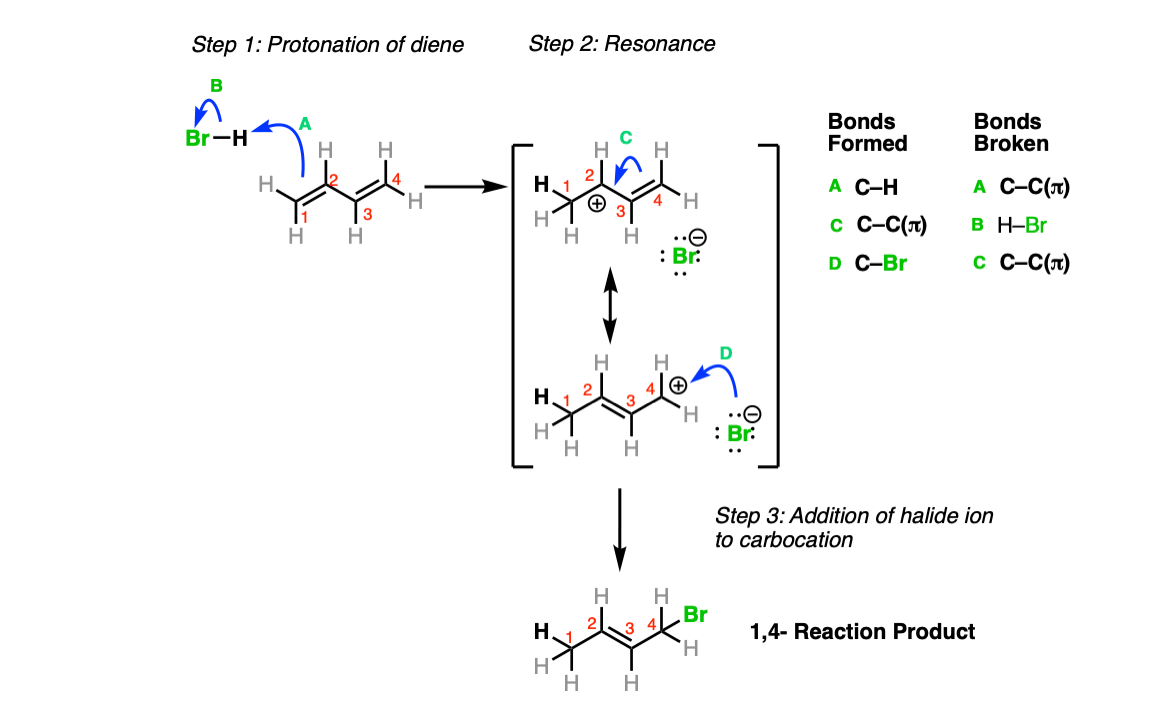
Notes: Although it’s helpful to draw out the two resonance forms and to draw the 1,4-attack at the bottom resonance form, keep in mind that resonance forms don’t equilibrate between each other – the “true” form is a hybrid between the two resonance forms.