Friedel-Crafts acylation of aromatic groups to give ketones
Description: In the presence of a catalyst such as FeCl3 or AlCl3 , acyl chlorides will add to aromatic groups to form aromatic ketones. This is called the Friedel-Crafts acylation reaction.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 2500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
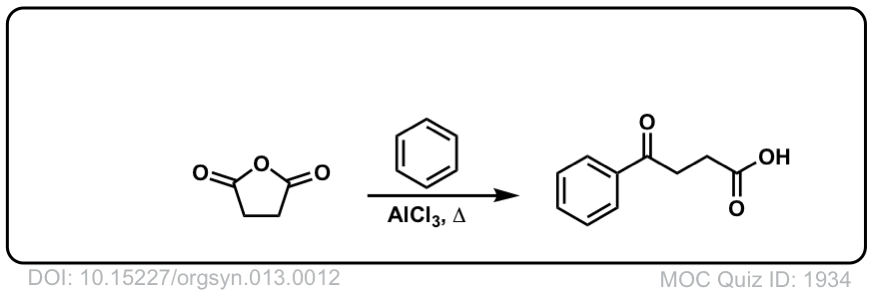
Org. Synth. 1933, 13, 12
DOI Link: 10.15227/orgsyn.013.0012
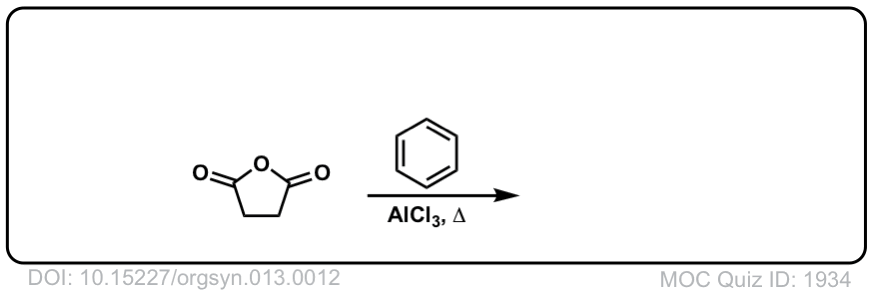 Click to Flip
Click to Flip
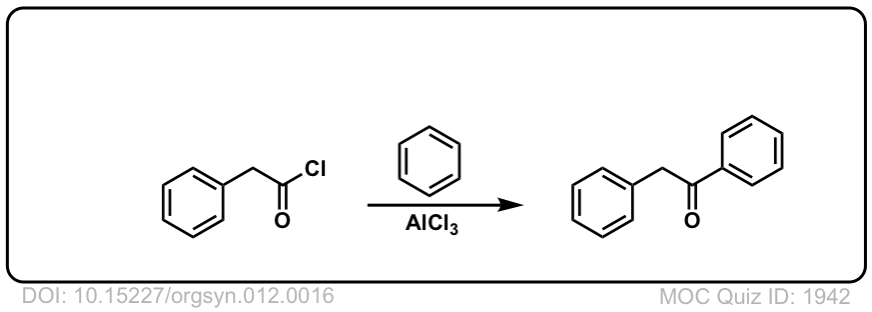
Org. Synth. 1932, 12, 16
DOI Link: 10.15227/orgsyn.012.0016
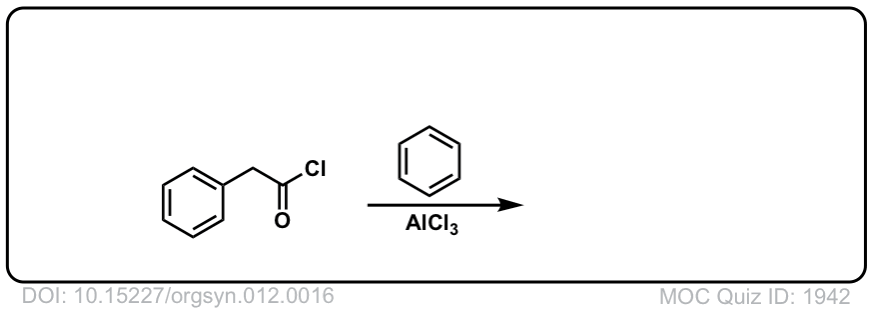 Click to Flip
Click to Flip
Org. Synth. 1934, 14, 40
DOI Link: 10.15227/orgsyn.014.0040
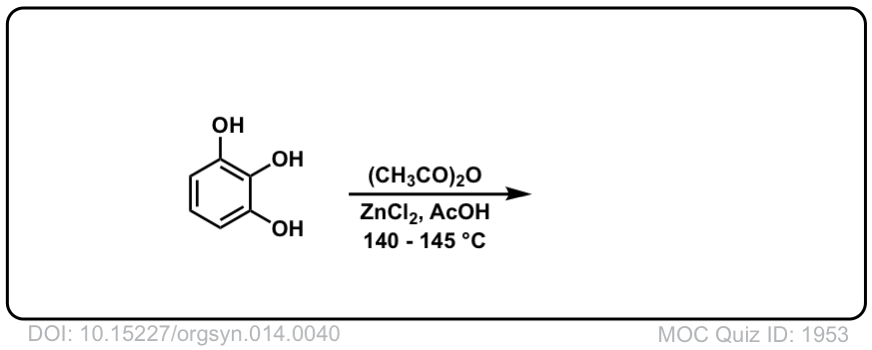 Click to Flip
Click to Flip
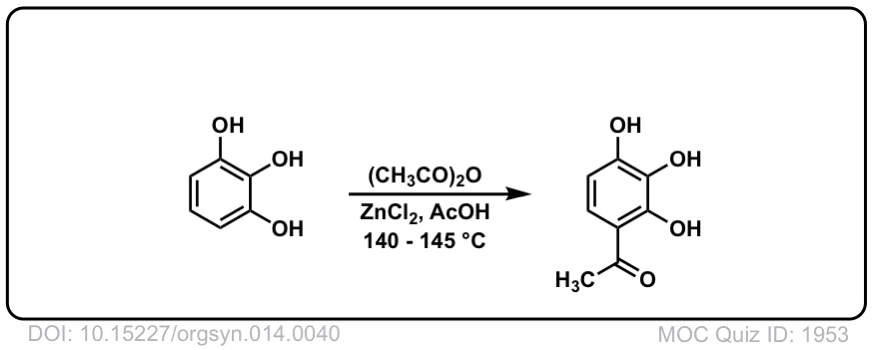
Org. Synth. 1935, 15, 77
DOI Link: 10.15227/orgsyn.015.0077
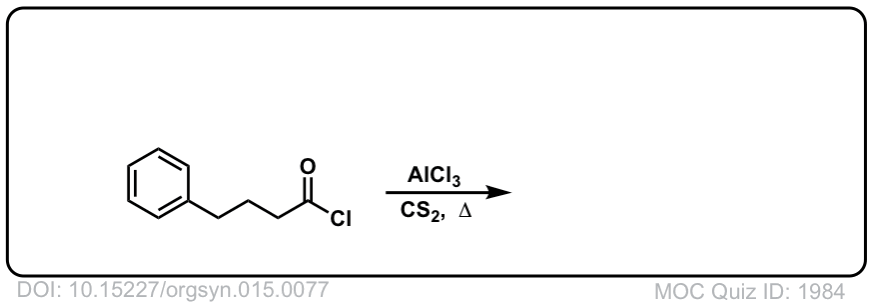 Click to Flip
Click to Flip
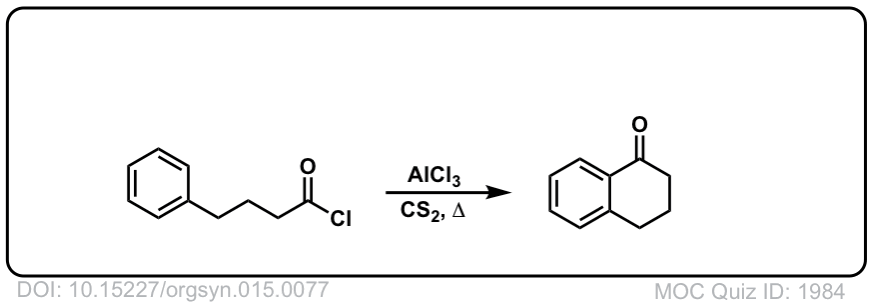
Org. Synth. 1940, 20, 1
DOI Link: 10.15227/orgsyn.020.0001
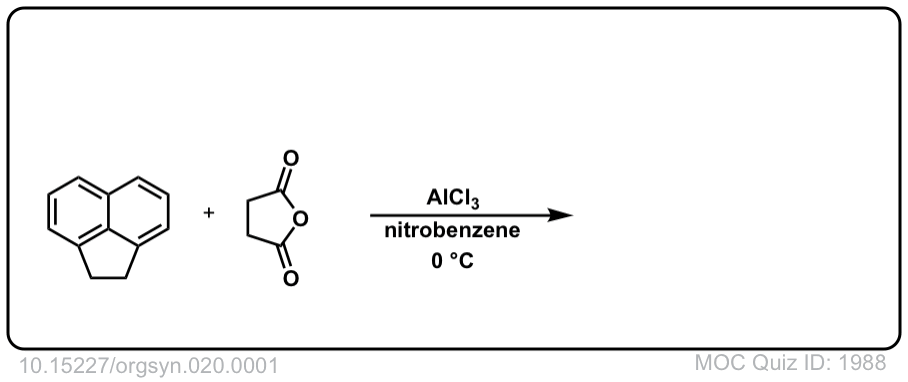 Click to Flip
Click to Flip
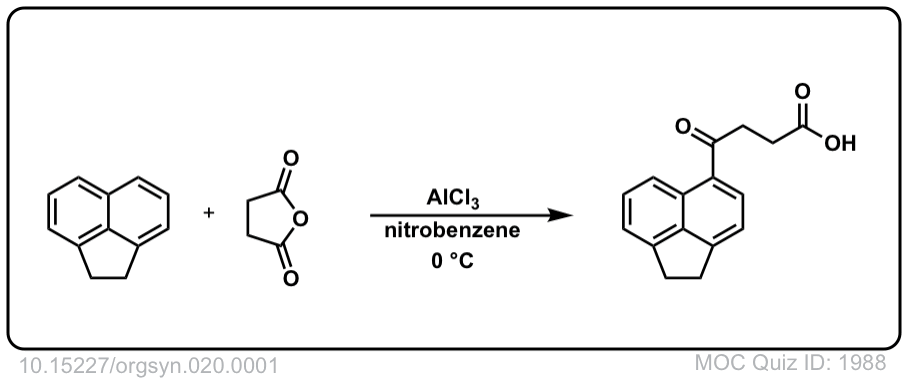
Org. Synth. 1940, 20, 94
DOI Link: 10.15227/orgsyn.020.0094
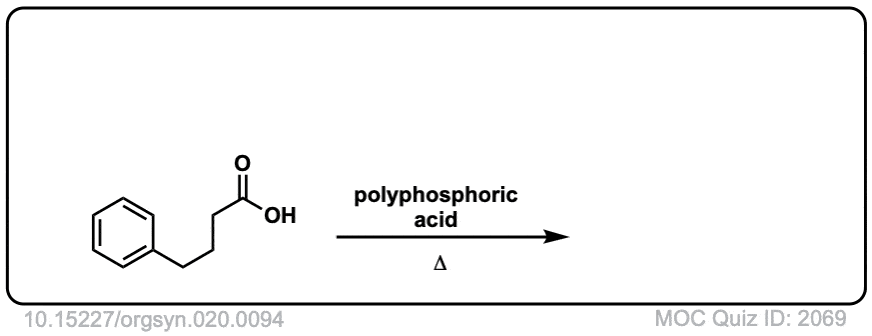 Click to Flip
Click to Flip
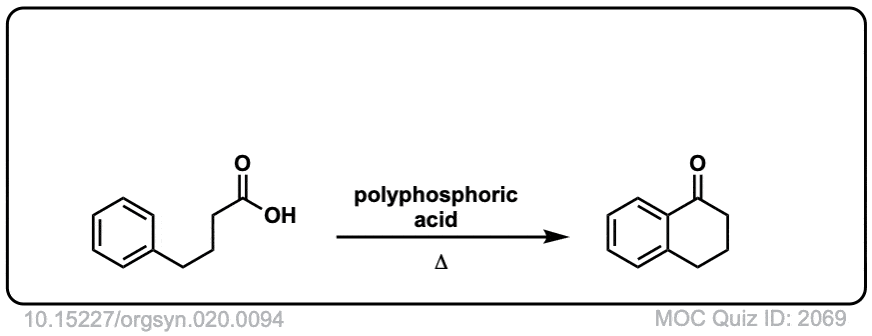
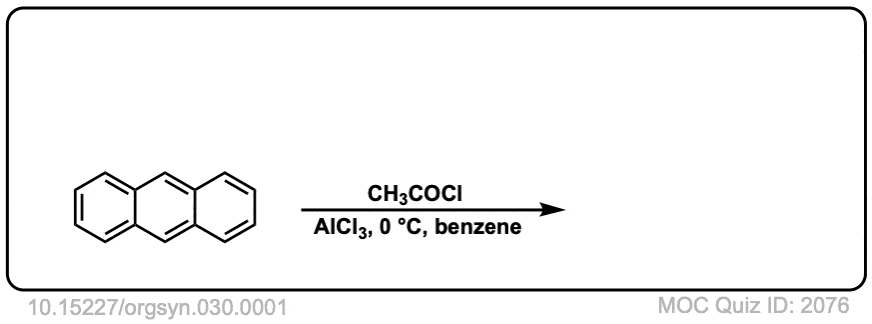
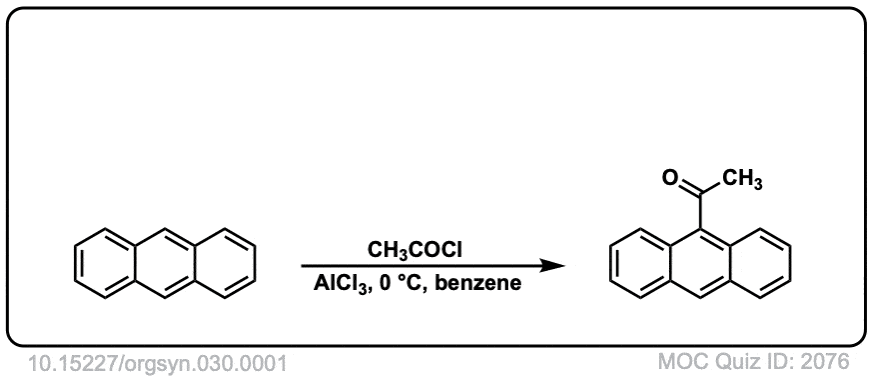
Org. Synth. 1950, 30, 1
DOI Link: 10.15227/orgsyn.030.0001
 Click to Flip
Click to Flip
Hello, I’m not sure but I think the arrows between acylium molecules should be resonance not equilibrium. Thank you.
Good catch. Generally resonance forms shouldn’t show movement of atoms (including bond rotation) but showing this as a resonance form is probably best!
Excuse me, there’s some thing really confused me.
Why benzophenone cannot under go Friedel-Crafts acylation?
Does it is for the reason that the carbonyl group is deactivating or that benzophenone has highly conjugated system to protect it from acylation or any other reasons?
Thank you very much.
I thought Cl was electron withdrawing group which is meta directing so why is product para directing
Cl is electron-withdrawing, but is an ortho-para director. https://www.masterorganicchemistry.com/2018/03/05/why-are-halogens-ortho-para-directors/
My textbook uses aluminum as the metal ion and you used iron (III). Does it matter what metal ion you use so long as the halide matches?
Can friedel-Crafts acylation be used to create an aldehyde. In other words, can the R group on the chloride acyl be a H?
Thats Gatterman Koch Reaction. It is based on F.C. Acylation
No you may not get benzaldehyde through FC. under the reaction conditions halodehydes will decompose forming hcl.
Can the R group be an alkene?
That would risk acylation of the alkene instead of the aromatic ring.
Hi there! I’m doing a problem in my book (don’t worry, it’s not graded hw), and I was wondering, would it be possible to add an anhydride with this reaction? Or would sterics/other reasons prevent that?