Darzens Condensation
Description: Alpha-halo esters treated with base can form enolates, which add to aldehydes or ketones. After addition, the resulting alkoxide can then form an epoxide with the adjacent carbon through an intramolecular SN2 reaction.
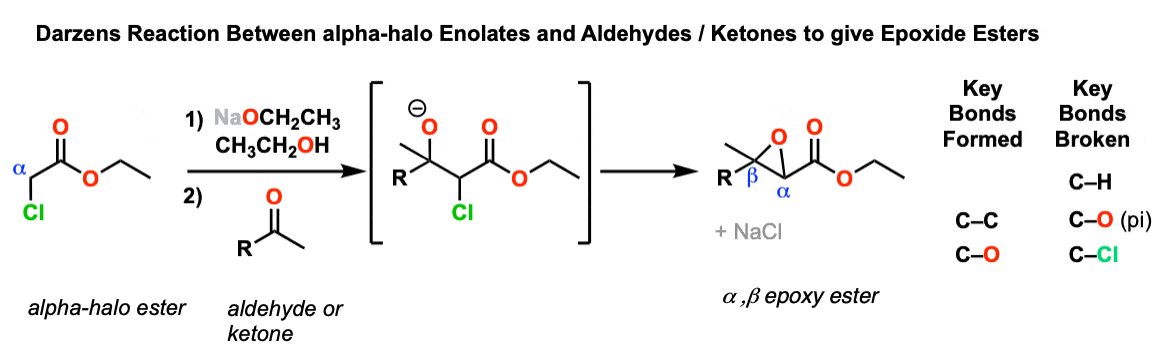
Notes: For obscure reasons, the products of these reactions are sometimes known as glycidic esters.
This is a lot like an aldol addition reaction followed by an intramolecular SN2.
See Also: Aldol addition reaction, Claisen condensation, Formation of epoxides with sulfur ylides
Examples:
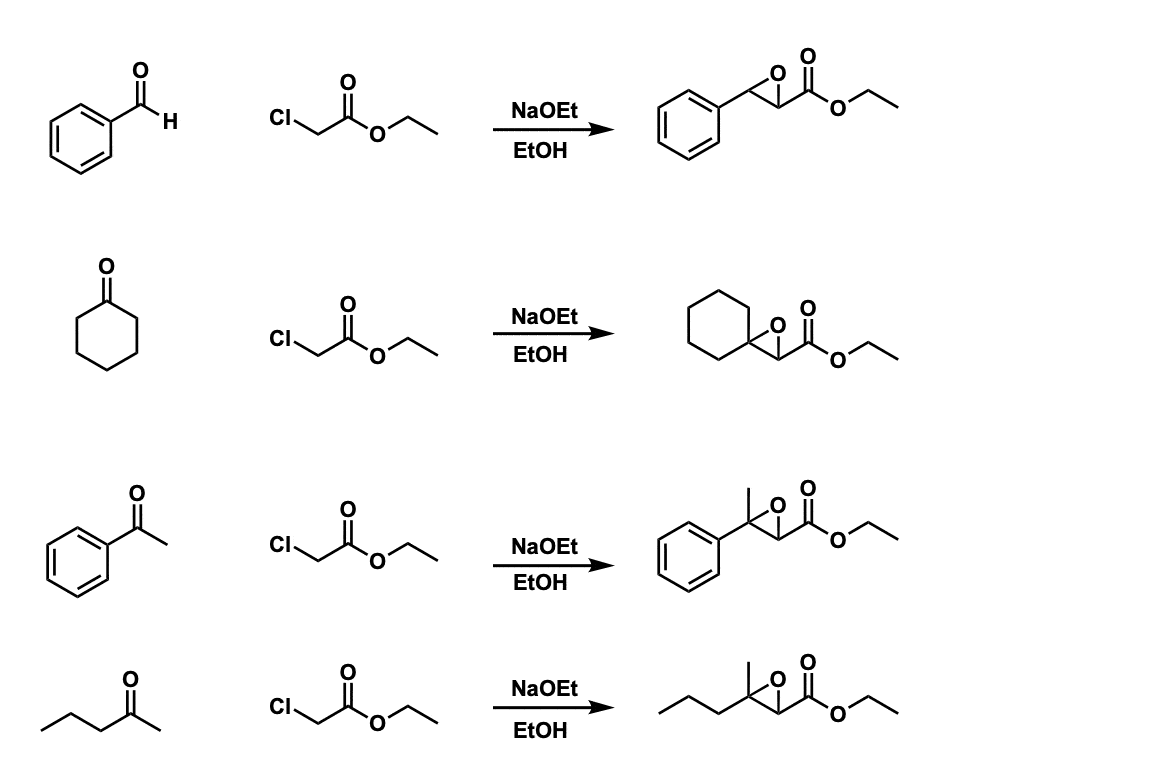
Notes:
The choice of base is usually the alkoxide corresponding to the ester. For instance, with an ethyl ester, sodium ethoxide is often used. (NaNH2 can be used also).
The reaction works best for aromatic aldehydes and ketones. It works less well for simple aldehydes (ethanal, for example, gives very poor yields, probably due to competition with self-aldol).
Mechanism:
The first step of the Darzens is deprotonation of the alpha-carbon of the alpha-chloro ester to give an enolate (Step 1, arrows A, B, and C). The enolate has a significant resonance form with a negative charge on carbon (arrows D and E).
Attack of the enolate carbon on the electrophile (here it is acetone) leads to formation of a new C-C bond and breakage of C-O (pi) (Step 2, arrows F and G).
This results in an alkoxide, which can then perform an intramolecular SN2 reaction to give an epoxide (Step 3, arrows H and I).
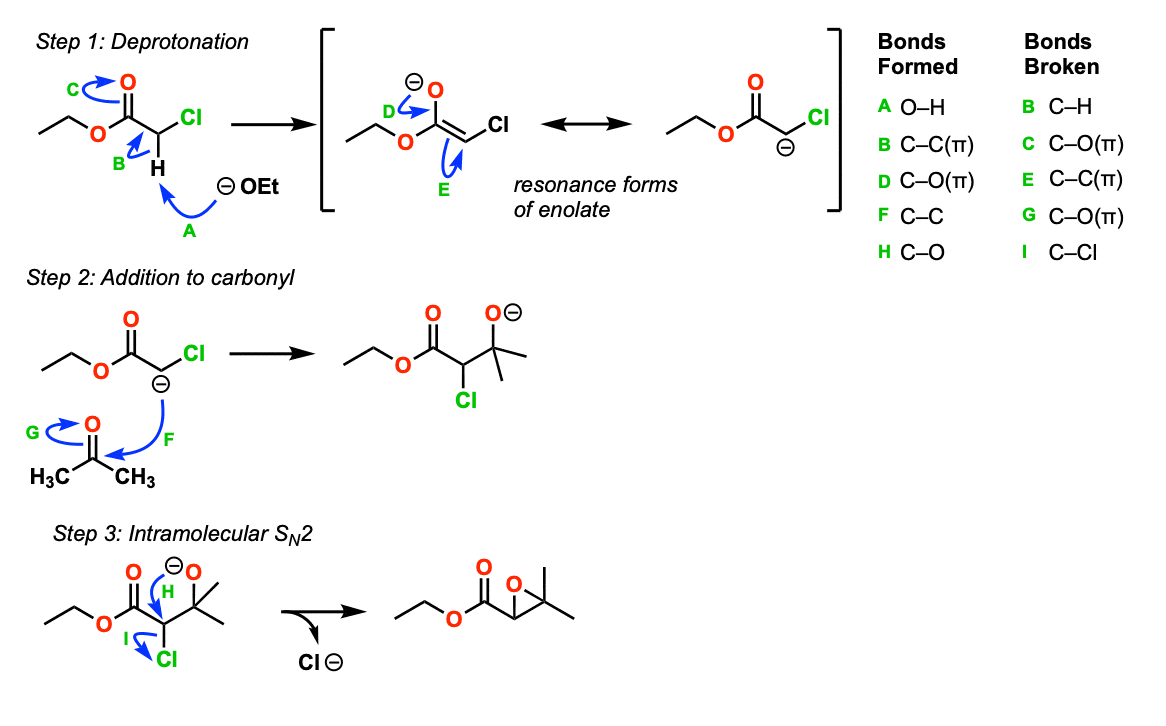
Notes: The last step is similar to the formation of epoxides from halohydrins, which also goes through an intramolecular SN2.
(Advanced) References and Further Reading
- The Darzens Glycidic Ester Condensation
Melvin S. Newman, Barney J. Magerlein
Organic Reactions. 5 (10): 413–440.
DOI: 10.1002/0471264180.or005.10.
Review from Organic Reactions. This has a good discussion of the history of the Darzens and its scope. The great thing about Organic Reactions is that it contains every single published example of a reaction up to that point. There is an appendix in this review that very clearly shows the yields of the Darzens reaction with a large variety of different aldehydes and ketones. - Mechanisms of The Darzens and Related Condensations
Manuel Ballester
Chemical Reviews 1955 55 (2), 283-300
DOI: 10.1021/cr50002a002
Discussion on the mechanism of the Darzens condensation.