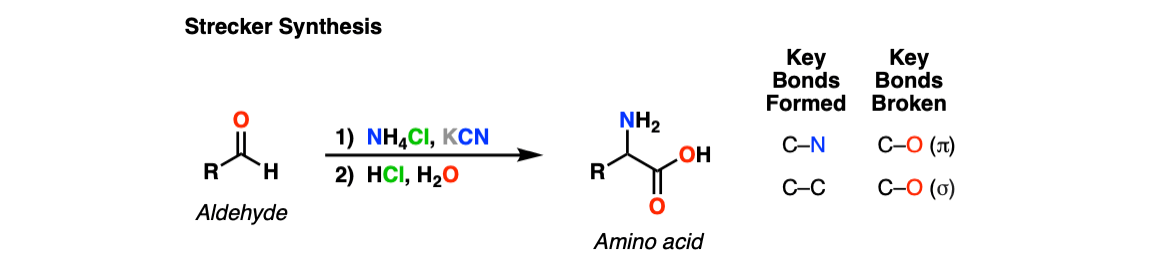
Strecker Synthesis
Description:
The Strecker Synthesis is a way of making amino acids from aldehydes, via 1) formation of an imine, 2) addition of cyanide ion to give an alpha-amino nitrile, and then 3) hydrolysis of the nitrile to give a carboxylic acid.
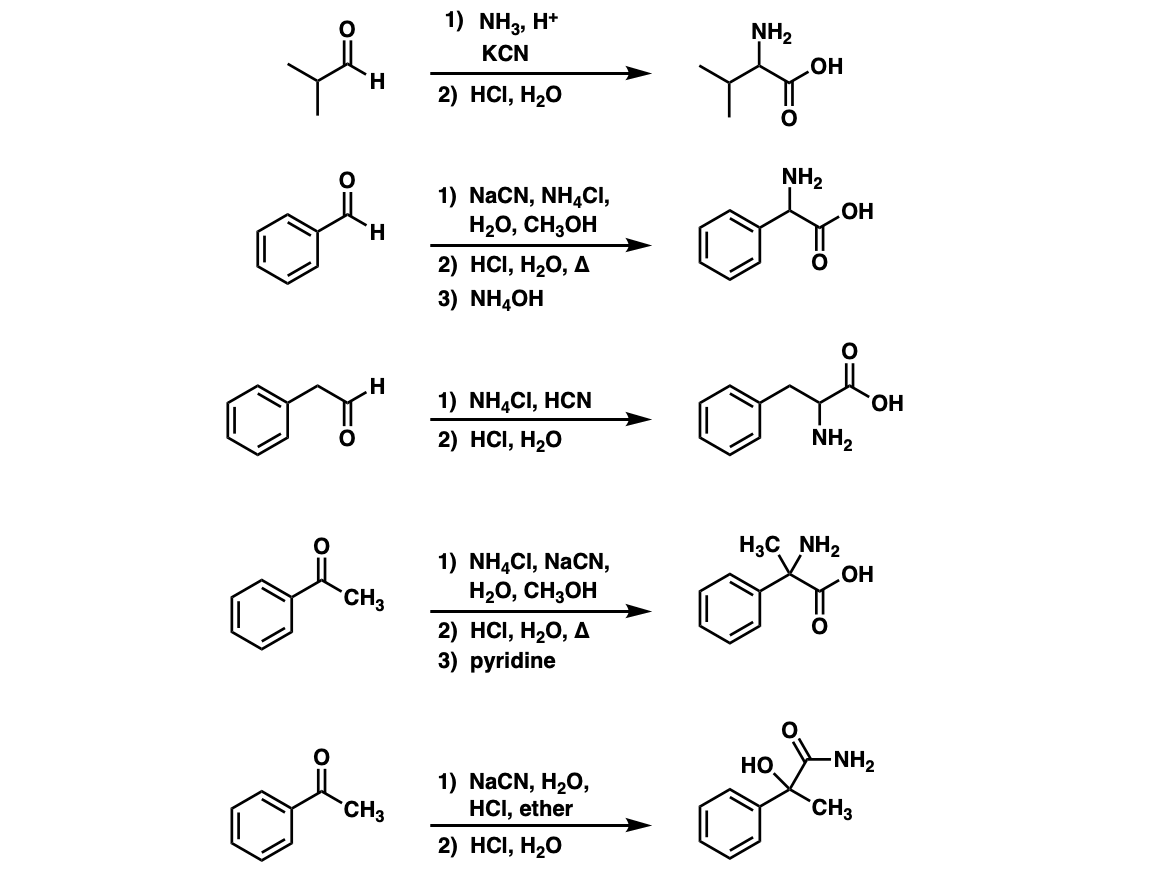
Notes: The NH4Cl serves as both a mild acid (pKaH of NH4+ is about 10) and a source of NH3, which converts the aldehyde to an imine. Once formed, the cyanide ion (here depicted as the potassium salt KCN) then adds to the imine. The purpose of aqueous acid is to hydrolyze the cyanide ion to the carboxylic acid.
Examples:
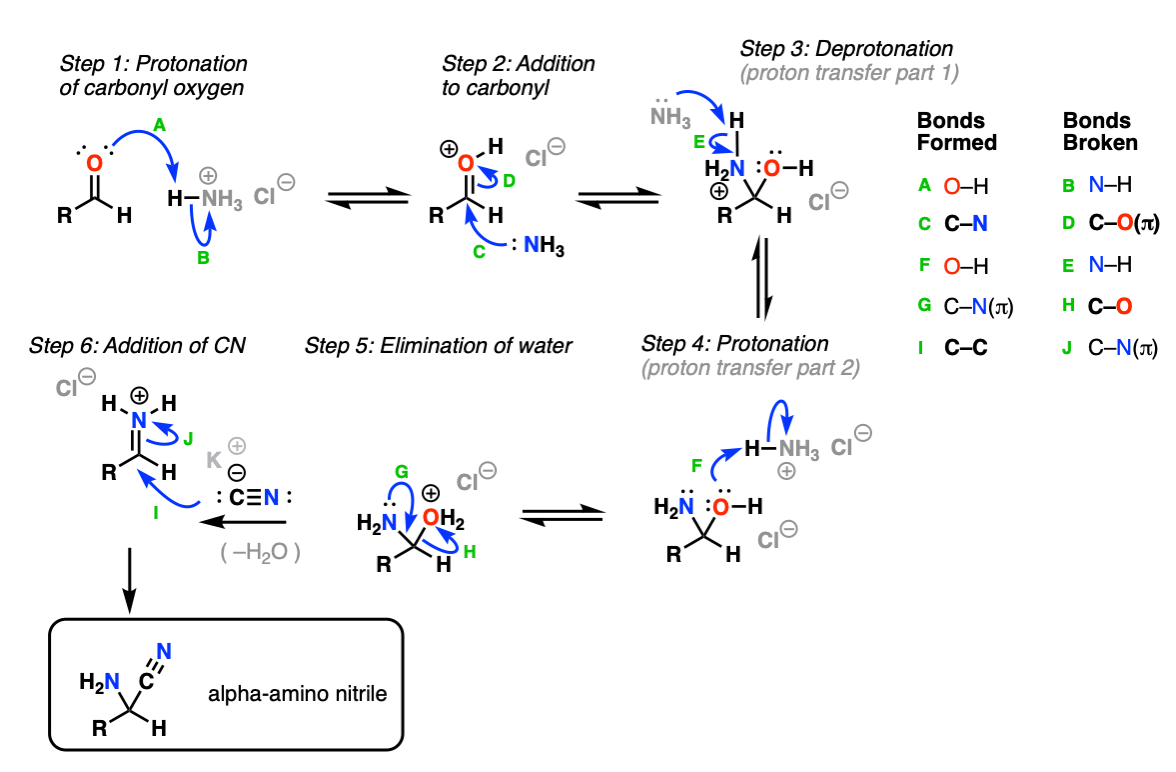
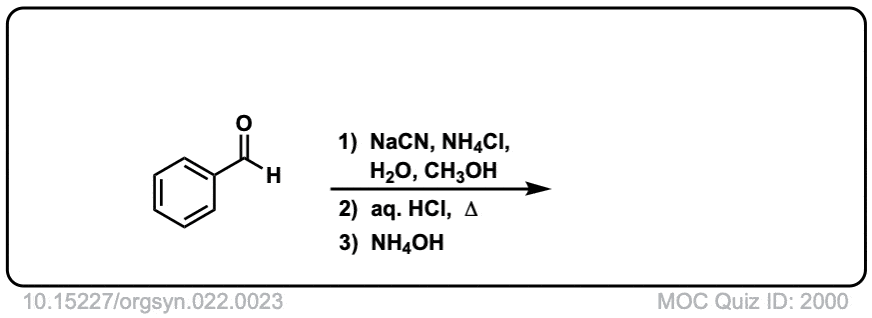
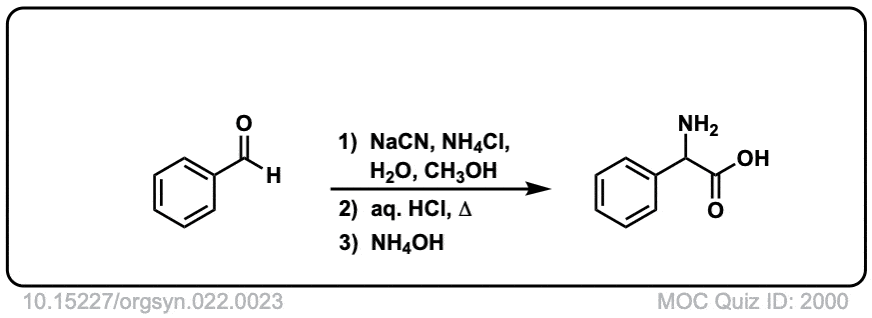
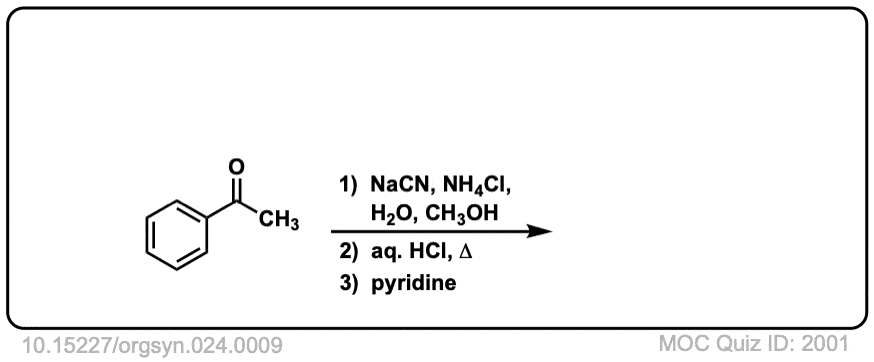
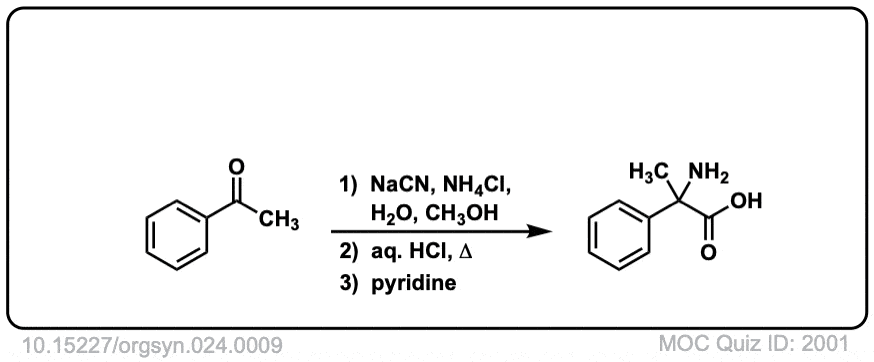
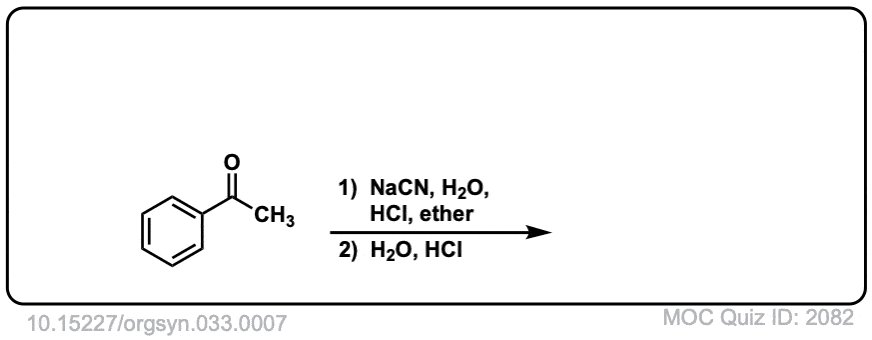
Notes: Examples 1, 2, and 3 are fairly typical Strecker reactions for the formation of various amino acids. Example 4 uses a ketone instead of an aldehyde. The “pyridine” in the third step (often omitted on questions) is to neutralize the amino acid after the hydrolysis is complete. Example 5 actually has no source of nitrogen in the first step, so -CN is adding to a ketone to give an alpha hydroxy carboxylic acid. (This is essentially the first step of the Kiliani-Fischer synthesis).
Mechanism (Part 1, Formation of Alpha-Amino Nitrile)
The Strecker occurs in two stages. The first stage is formation of the alpha-amino nitrile from the aldehyde/ketone, ammonia (NH3) and cyanide ion.
NH4Cl is a weak acid, and can protonate the aldehyde (Step 1, arrows A and B) which is a better electrophile than the aldehyde itself. Next, NH3 (which is in equilibrium with NH4Cl under these conditions) adds to the protonated aldehyde (Step 2, arrows C and D) giving a tetrahedral intermediate.
Proton transfer (Steps 3 and 4, arrows E and F) results in OH2(+) which is a good leaving group. Elimination of water (Step 5, arrows G and H) results in a new iminium ion, which is then attacked with the cyanide ion (Step 6, arrows I and J) forming the new C-C bond and giving the alpha-amino nitrile.
Mechanism (Part 2, Hydrolysis of the Nitrile To Give The Amino Acid)
The second part of the Strecker synthesis is hydrolysis of the nitrile to give a carboxylic acid. (See Hydrolysis of Nitriles).
In the first step (Step 1, arrows A and B) the nitrile nitrogen is protonated (note that the amine will almost certainly be protonated here too, but we’re trying to keep things simple). Water then adds to the protonated nitrile (Step 2, arrows C and D) which undergoes proton transfer from oxygen to nitrogen (Steps 3 and 4, arrows E and F). The protonated imine is a good electrophile which can then undergo addition by a second equivalent of water (Step 5, arrows G and H). A second proton transfer event occurs, from oxygen to nitrogen (Steps 6 and 7, arrows I and J). The good leaving group NH3 is then eliminated (Step 8, arrows K and L) and the protonated carboxylic acid is deprotonated (Step 9, arrows M and N) to give the new amino acid.
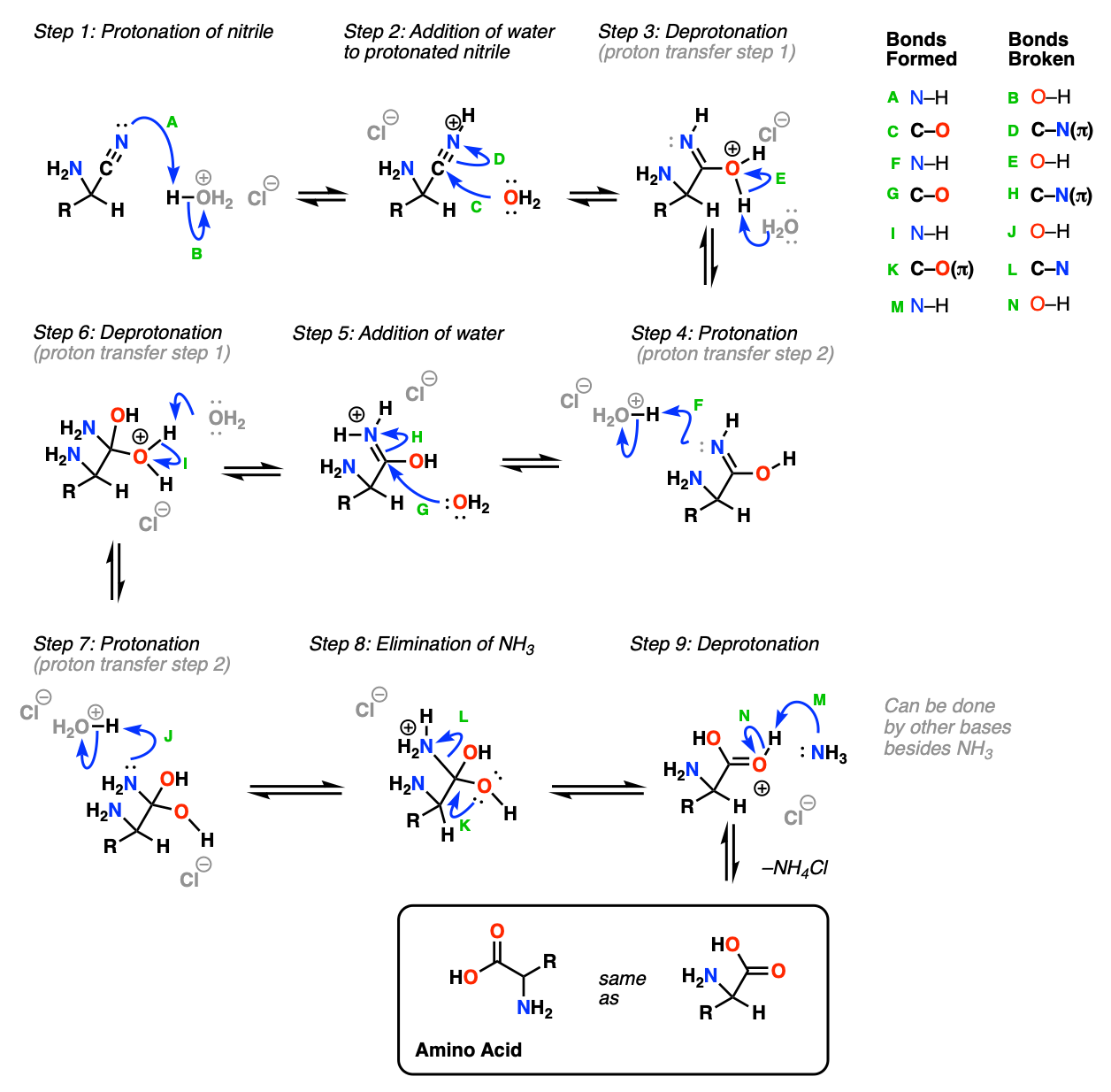
Notes: In practice, under the acidic conditions used, the final amino acid product will actually have a protonated amine nitrogen and will need to be neutralized.
Real-Life Examples:
Org. Synth. 1942, 22, 23
DOI Link: 10.15227/orgsyn.022.0023
 Click to Flip
Click to Flip
Org. Synth. 1944, 24, 9
DOI Link: 10.15227/orgsyn.024.0009
 Click to Flip
Click to Flip
Org. Synth. 1953, 33, 7
DOI Link: 10.15227/orgsyn.033.0007
 Click to Flip
Click to Flip