A Primer On Organic Reactions
What Makes A Good Leaving Group?
Last updated: March 4th, 2025 |
Good Leaving Groups Are Weak Bases
- A leaving group (a.k.a. “nucleofuge”) is the new Lewis base that is generated in various substitution and elimination reactions when a new bond is formed to carbon.
- Just as acid-base reactions favor reactions where a stronger acid plus a stronger base results in a weaker acid and a weaker base, substitution and elimination reactions tend to favor reactions where the leaving group is a weaker base than the nucleophile (or base, in the case of elimination reactions)
- Using pKa values it is possible to make good predictions as to whether various substitution or elimination reactions are favorable. Good leaving groups tend to be weak bases, i.e. the conjugate bases of strong acids (I–, Br–, Cl–, TsO–, H2O)
- Poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a strong acid (or a Lewis acid). For example, alcohols can be converted into alkyl chlorides through addition of HCl.
- Although the relative basicity of the nucleophile/leaving group is a good guide, a prominent exception is fluoride (F-) which is a weak base but tends not to act as a leaving group due to the extremely strong C-F bond (about 130 kcal/mol).
- When the basicity of the nucleophile and leaving group are comparable (i.e. within about 5-10 pKa units), an equilibrium may be established.
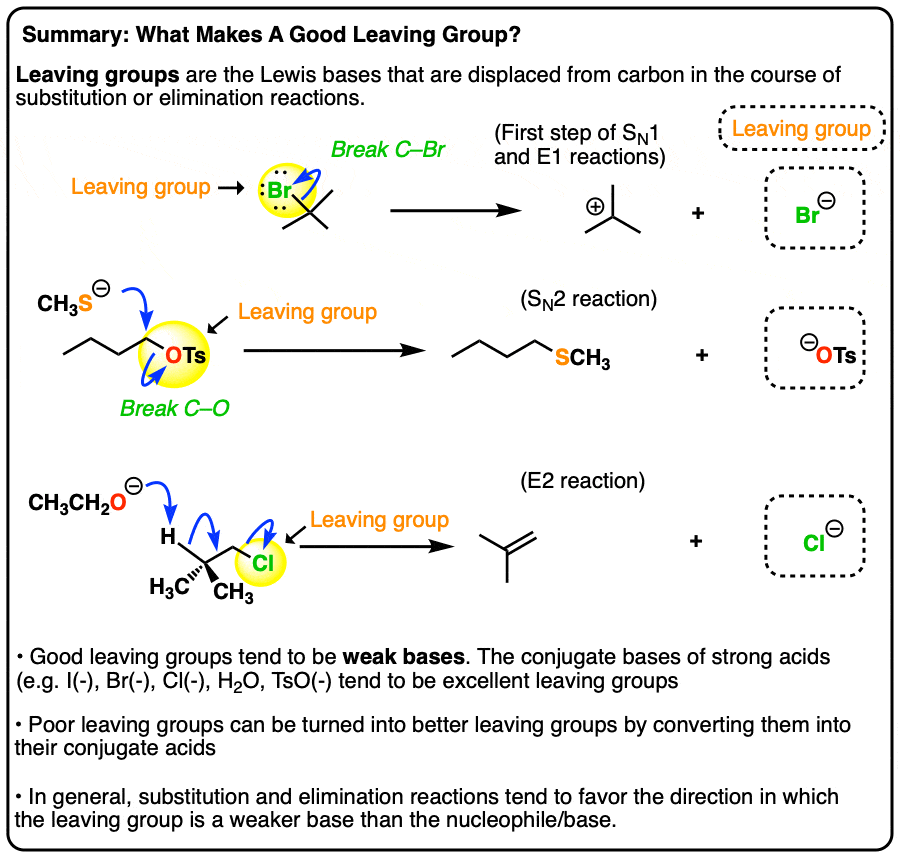
Table of Contents
- What Is A Leaving Group?
- The Principle of Acid-Base Mediocrity, Revisited
- Using a pKa Table To Evaluate Leaving Group Ability
- The Conjugate Acid Is A Better Leaving Group
- The Conjugate Base Is A Worse Leaving Group
- Exceptions – Fluorine
- The Best Leaving Group of All (N2)
- Summary
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. A Leaving Group Is Just A Nucleophile Going In Reverse
Many reactions that involve nucleophiles and bases result in the formation of a leaving group (also called a “nucleofuge“).
So what is a leaving group, anyway?
Well, a leaving group is just a nucleophile acting in reverse.
That is, whereas a nucleophile is the Lewis base that forms a new bond with a carbon, the leaving group is the Lewis base that results after breaking a bond with the carbon.
Here is an example of a reaction that produces a leaving group. This is a nucleophilic substitution reaction – specifically a nucleophilic substitution reaction at an alkyl carbon with with a bimolecular rate-determining step (SN2).
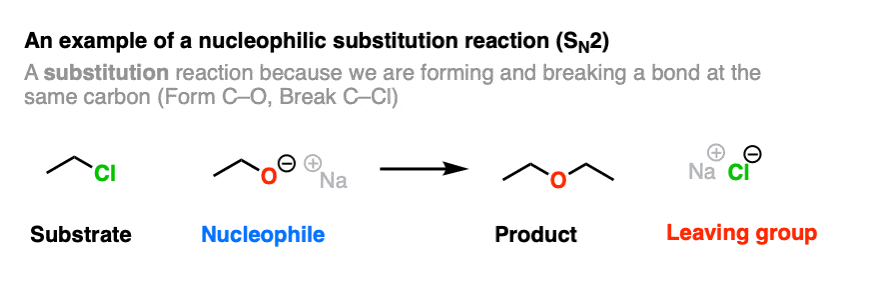
Note that this reaction has four components.
- A substrate (an alkyl halide in this case, which will be acting as an electrophile)
- A nucleophile (the CH3CH2O(-) ion)
- The product (the ether)
- The leaving group (the chloride ion, Cl(-) )
A C-O bond is formed, and a C-Cl bond is broken.
To see a few more examples of reactions that result in leaving groups we will cover below, hover here for a pop-up image or click on this link.
There is nothing stopping us from taking the reaction drawn above and flipping it around to give a reaction that goes in the opposite reaction. So let’s draw that out:
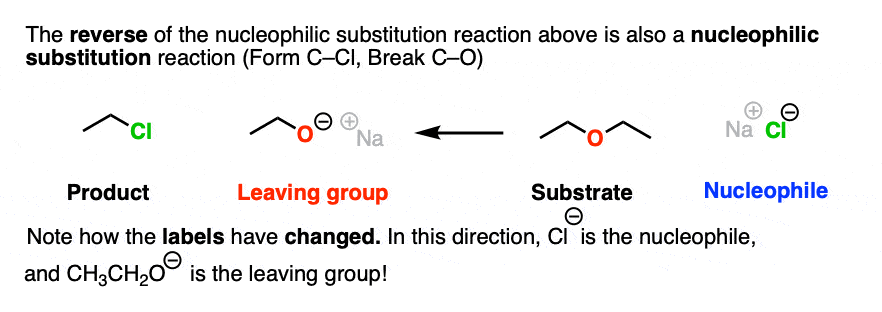
Note how in this drawing, the Cl(-) is the nucleophile and the RO(-) is the leaving group.
Note that I’m not saying that these two reactions are equally likely. Just because we can draw these two reactions does not mean that they are equally favored in a reaction vessel.
However, it is true that, as drawn, both of these reactions are nucleophilic substitution reactions, because we are forming and breaking a bond on the same carbon.
That being said: which reaction do you think is more favored?
In other words, if we ran the experiments, which reaction would you predict to proceed to completion?
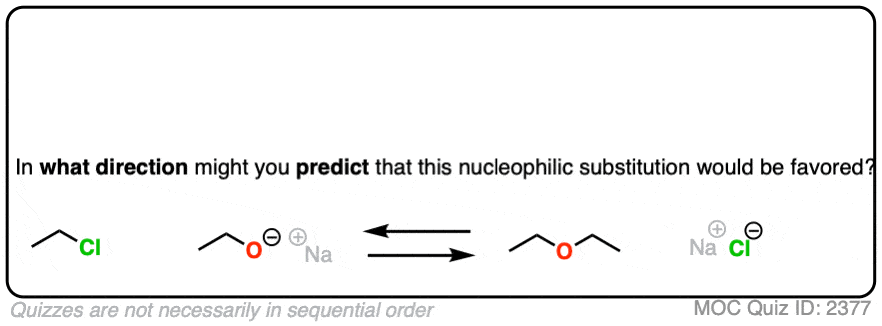 Click to Flip
Click to Flip
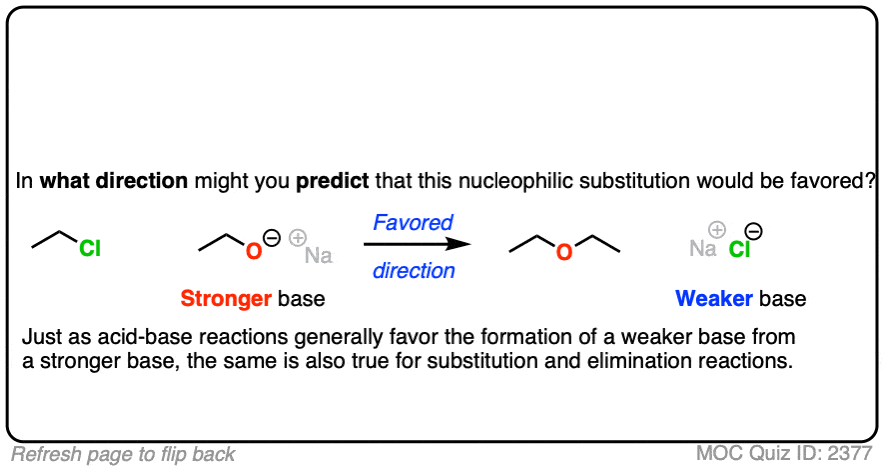
Congratulations if you said, the top one.
Why?
2. Are Substitution and Elimination Reactions Just Fancy Extensions of Acid Base Reactions?
Let’s change one little thing about that reaction above, and see if this looks a bit more familiar.
In the acid-base reaction between H-Cl and NaOCH2CH3 to give CH3CH2OH and NaCl, which direction would be more favored?
You might recall the Principle of Acid-Base Mediocrity: Stronger acid plus stronger base gives weaker acid and weaker base. (See post: How To Use a pKa Table).
So the favored direction of this acid-base reaction is formation of the weaker base (Cl -)
Just as acid-base reactions tend to be favored when we go from a stronger base to a weaker base, substitution and elimination reactions also tend to be favored when the leaving group is a weaker base than the nucleophile (or base).
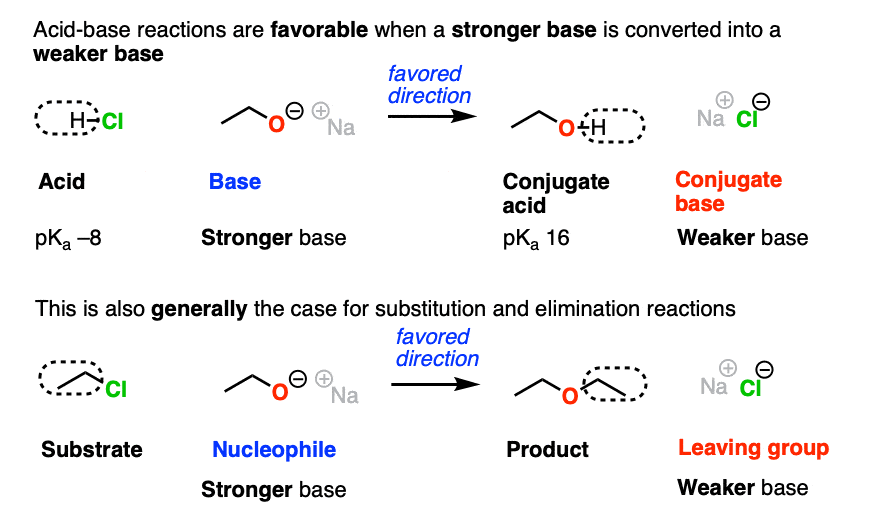
In fact, the four components of an acid base reaction map pretty well onto the four components of the nucleophilic substitution reactions, above.
- Acid → substrate
- Base → nucleophile
- Conjugate acid → product
- Conjugate base → leaving group
The substitution above also favors formation of the weaker base (Cl-) from the stronger base (CH3CH2O-)
For that matter, so does this elimination reaction
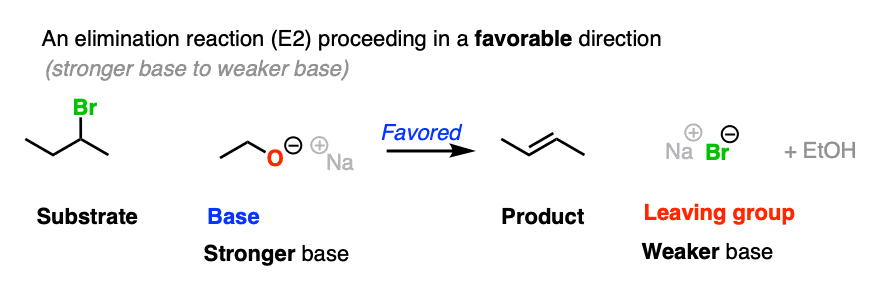
If the leaving group and base are flipped, the reaction is much less favored. To see this “flipped” reaction, hover here for a pop-up image or click on this link.
This is also the case with the loss of leaving group in this SN1 reaction:
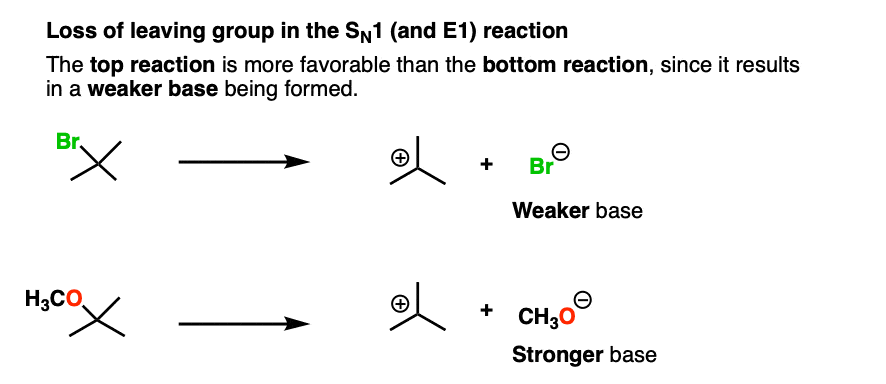
There are many more examples, like these nucleophilic acyl substitution reactions that you will see in Org 2. To see an example, hover here for a pop-up image or click on this link.
Generally speaking, the weaker the base, the better the leaving group.
We can reverse this and also say that the stronger the base, the worse the leaving group.
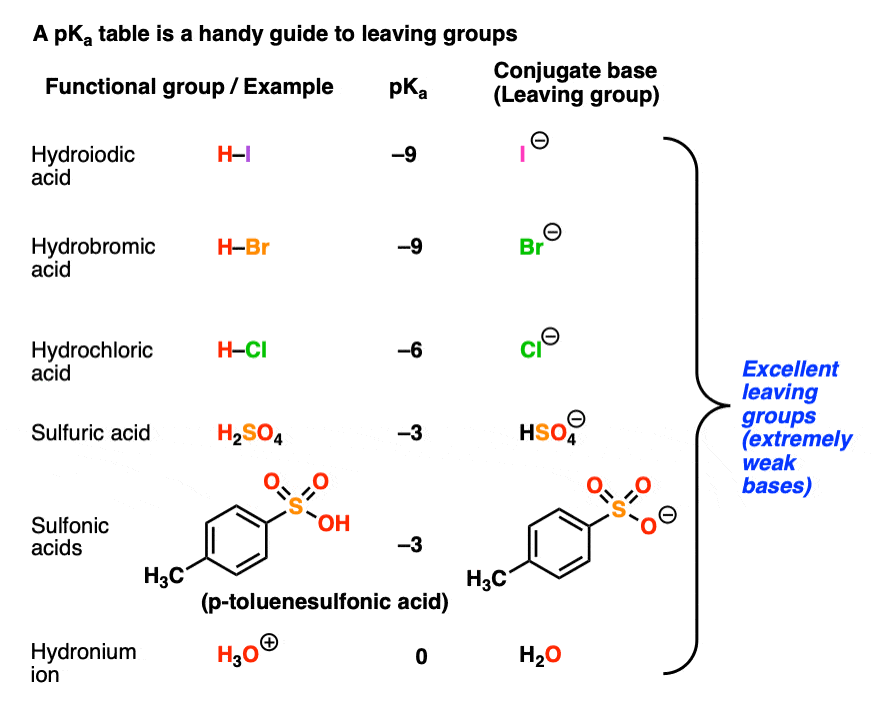
3. Using a pKa Table To Determine Leaving Group Ability
If base strength determines leaving group ability, then a pKa table should be a pretty good guide to determining leaving group ability.
In a previous post (See: How To Use a pKa Table) we went through this in considerable detail.
Recall that the stronger the acid, the weaker the conjugate base.That means that the top portion of a pKa table should be an excellent guide to the best leaving groups. The conjugate bases of strong acids, such as I(-), Br(-), Cl(-), HOSO3(-), TsO(-), H2O, and others are weak bases and great leaving groups.
Here is another example of a favorable reaction, where the nucleophile is more basic than the leaving group:
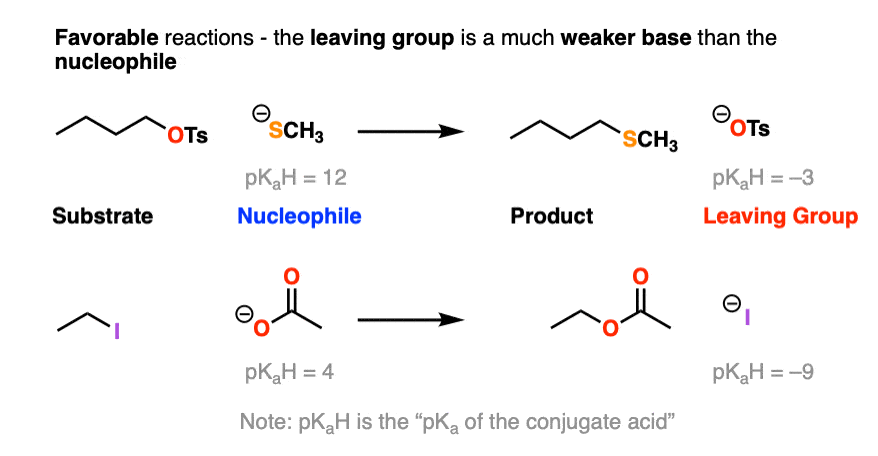
Note that pKaH is shorthand for “the pKa of the conjugate acid”.
On the other hand, the bottom part of a pKa table should be a guide to the worst leaving groups – the conjugate bases of weak acids, such as H(-) and H3C(-) are extremely strong bases.
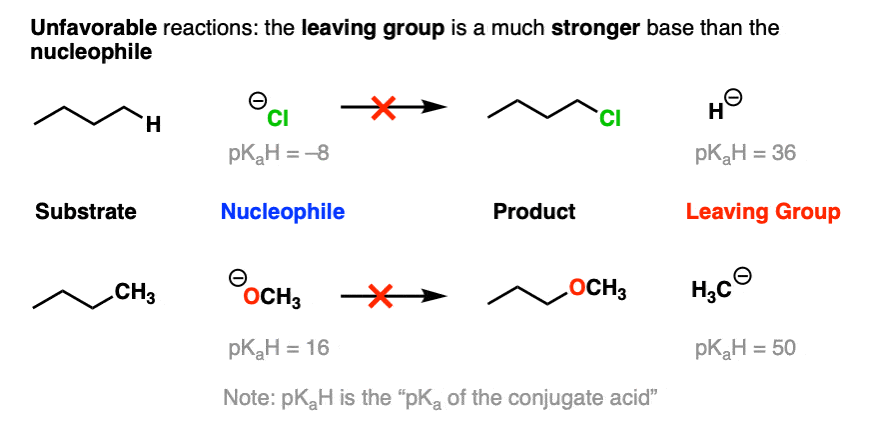
This is why we never see alkyl groups or hydride ions as leaving groups.
I should stress that comparing the basicity of the nucleophile and leaving group is a good place to start, but is not the only variable. The relative strength of the bonds being broken and formed is also a factor, which comes up most prominently with fluorine (see Exceptions, below).
When the nucleophile and leaving group are of comparable basicity (i.e. within about 5-10 pKa units), substitution reactions are potentially reversible, and the equilibrium can be driven to one side or the other depending on what conditions we choose. [Note 1]
Also, it’s important to remember that acidity and basicity (i.e. pKa) are measured by the position of an equilibrium (think “differences in energy”) whereas nucleophilicity is measured by reaction rates since many reactions of nucleophiles are irreversible. So although the correlation is very good, it isn’t perfect.
Also, be on the lookout for acid-base reactions. A good rule of thumb is that acid-base reactions are fast, relative to substitution and elimination reactions (see post: Acid-Base Reactions are Fast)
4. The Conjugate Acid Is A Better Leaving Group
Hydroxide ions are poor leaving groups because they are strong bases.
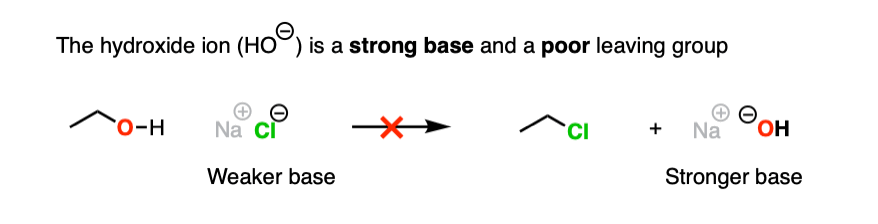
We might ask ourselves: is there some way to modify the OH so that when the C-O bond breaks, the leaving group is a weaker base? Then it would be more favorable.
Yes, there is a simple and effective way to make OH a better leaving group.
If we just add strong acid, then we make the conjugate acid of R-OH, which is R-OH2(+).
Now, if Cl(-) attacks, our leaving group will be the weak base H2O. This is a factor of 1014 less basic than HO(-).
So in the presence of acid, this reaction becomes much more favorable. The conjugate acid is a better leaving group! (This is one of the major applications of acid catalysis in organic chemistry.)
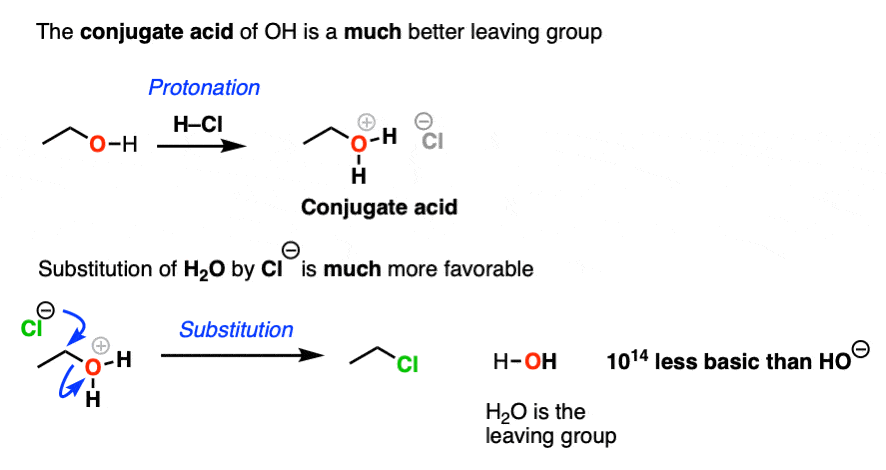
In the chapter on alcohols, we’ll see that many substitution and elimination reactions of alcohols require acid in order for the OH group to leave.
Lewis acids can be used for this purpose as well. [Note 2] We will see many examples of this in the chapter on electrophilic aromatic substitution (e.g. in the Friedel-Crafts reaction where Lewis acids like AlCl3 are used to make Cl into better leaving groups (See post: The Friedel Crafts Alkylation and Acylation Reactions).
Other ways to modify -OH to make it into a better leaving group includes transforming them into sulfonates, which are much weaker bases than HO(-). [See post: Tosylates and Mesylates] [Note 3]
5. The Conjugate Base Is A Worse Leaving Group
It’s true that the conjugate acid is a better leaving group. It’s also worth noting that the conjugate base is a worse leaving group.
Also, acid-base reactions are faster, generally speaking, than substitution and elimination reactions.
If a strong base is added to a species with an acidic proton, the acid-base reaction will occur first. This will give the conjugate base, which will possess a much worse leaving group than the neutral species.
Here’s an example. You might think the leaving group here is HO(-) . It’s not. The leaving group would have to be O(2-)
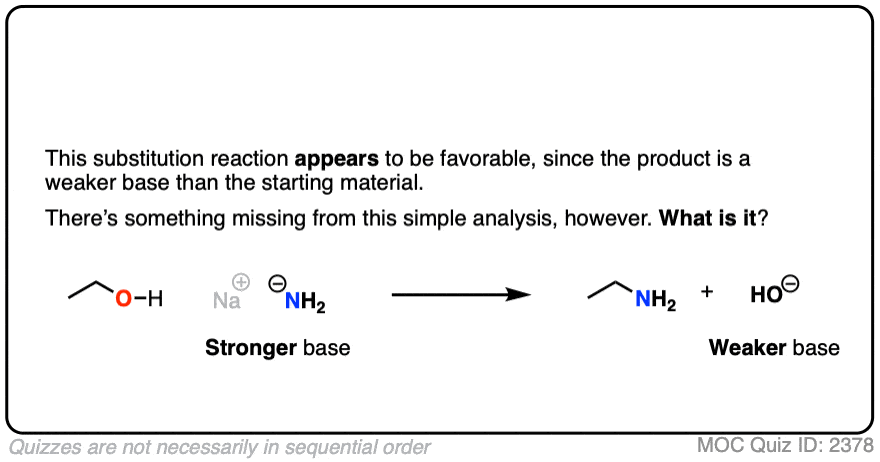 Click to Flip
Click to Flip
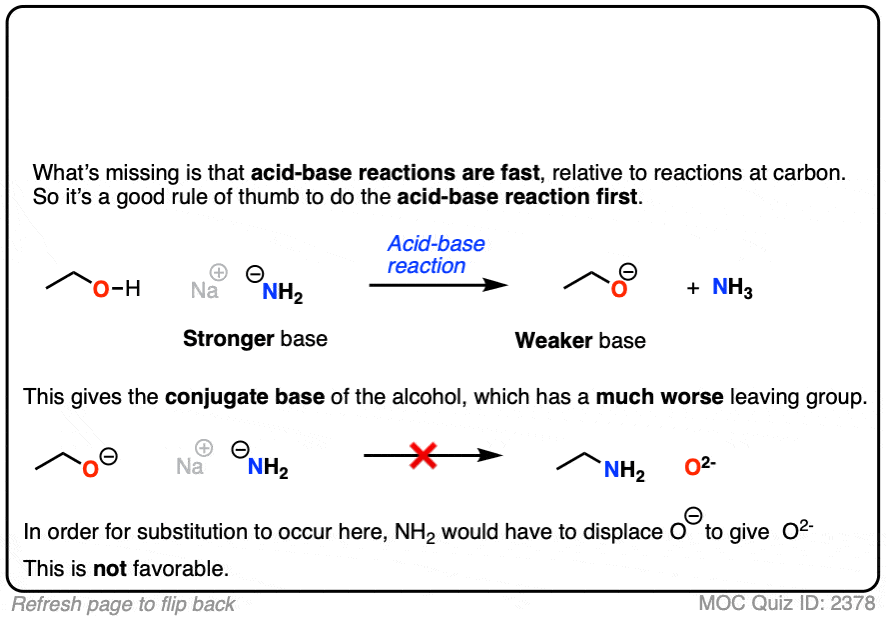
That’s a much worse leaving group.
Along the same lines, don’t forget that carboxylic acids…. are acids!
6. Exceptions
Is that all there is to it? Just apply the Principle of Acid-Base Mediocrity and we’re done with it?
Not quite. It wouldn’t be organic chemistry if we didn’t mention some exceptions.
One prominent exception is fluorine. Fluoride ion (F-) is a weak base (the pKa of HF is 3.8) , but generally a poor leaving group in substitution reactions. [Note 4]
One key reason is the strength of the C-F bond (about 130 kcal/mol).
Up to this point, we’ve only really considered the stability of the leaving group relative to the nucleophile. But the stability of the product is important as well.
Since the C-F bond is so strong, the reverse reaction (breaking the C-F bond, giving F(-) as a leaving group) would have an extremely high activation energy, making it very unfavorable.
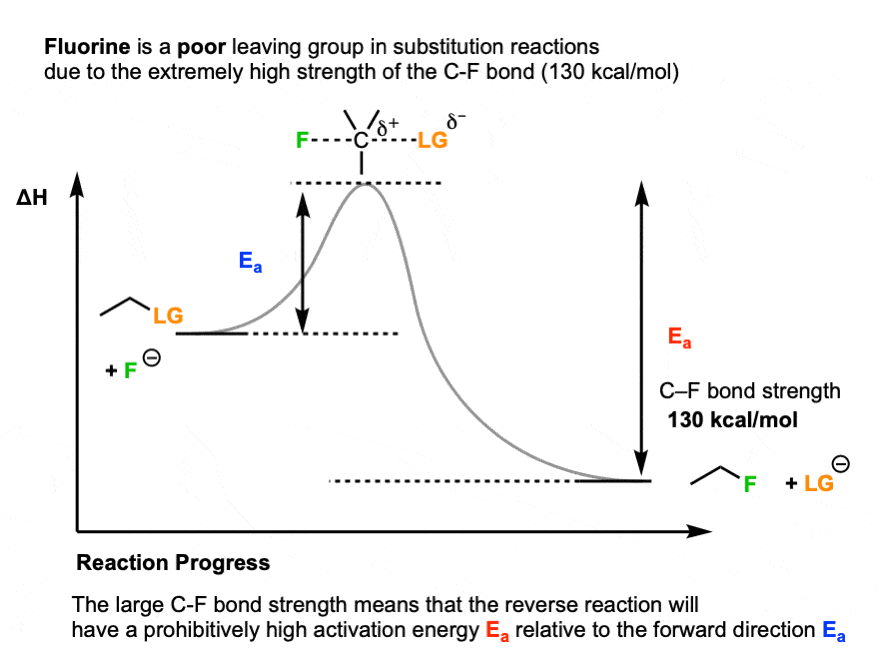
So the key variable is not just the relative stability of the nucleophile versus the base, but also the strength of the bond that is forming and breaking.
Another example of a reaction where fluorine is a leaving group is nucleophilic aromatic substitution, but the breaking of C-F is not the rate-determining step in this case (See Post: Nucleophilic Aromatic Substitution)
7. The Best Leaving Group Of All
Probably the best leaving group of all is dinitrogen (N2). Not only is nitrogen exceptionally stable – after all, it’s 79% of our atmosphere and is essentially inert – it is a gas, which means that any organic reaction where N2 is displaced will result in it bubbling out of solution, rendering the reaction essentially irreversible (sometimes explosively so!).
Amines (R-NH2) can be converted into diazonium salts R-N≡N(+) with nitrous acid (HONO) through a process called “diazotization”.
However dinitrogen is such a good leaving group that sp3-hybridized carbon atoms bonded to N2 are exceptionally unstable and tend not to stick around long enough to participate in reactions with nucleophiles; once formed, the N2 just leaves, resulting in carbocations (and not uncommonly, shattered glassware) in its wake.
Diazonium salts of sp2-hybridized carbons are considerably more stable and used in organic reactions; for more information, see this post (See post: Reactions of Diazonium Salts)
8. Summary
Let’s review.
- Leaving groups are nucleophiles acting in reverse – i.e. they are Lewis bases formed from the breakage of a bond to carbon.
- Just as acid-base reactions are favored in the direction which results in a weaker base being formed from a stronger base, reactions involving leaving groups also tend to be favored in the direction which gives the weaker base.
- The conjugate bases of strong acids (e.g. I-, Br-, Cl-, TsO-, H2O) are good leaving groups.
- The conjugate base of weak acids (H-, H3C(-), alkyl anions) are poor leaving groups.
- The conjugate acid is a better leaving group. One common example is with alcohols, which can undergo substitution and elimination reactions after the HO is protonated.
- The conjugate base is a worse leaving group. A good rule of thumb is to do acid-base reactions first.
- Fluoride ion (F-) is a weak base, but a poor leaving group due to the strength of the C-F bond.
If you’re not sure where a reaction is going to happen on a molecule, look for a good leaving group. That’s usually where the action is!
Notes
Note 1. When the basicity of the nucleophile and the leaving group are comparable (i.e. within about 5-10 pKa units) then the reaction has the potential to be reversible. (For more information on this 5-10 pKa unit number, see A Handy Rule of Thumb For Acid-Base Reactions)
In this case it is an equilibrium and the position of the equilibrium can be influenced by Le Chatelier’s principle. For example it is possible to make a reaction proceed from a weaker base to a stronger base by using a large excess of the nucleophile, or by removing the stronger base.
One interesting example is based on different solubilities. The Finkelstein reaction is a way of making alkyl iodides from alkyl chlorides. There is not a tremendous difference between the leaving group ability of chloride ion or iodide ion. However, there is a significant difference between the solubility of NaCl (or NaBr) and NaI in acetone. NaI is soluble in acetone; NaCl is not. Therefore as NaCl is formed, it precipitates out of solution, driving the reaction to the right. Another example of Le Chatelier’s principle in action!
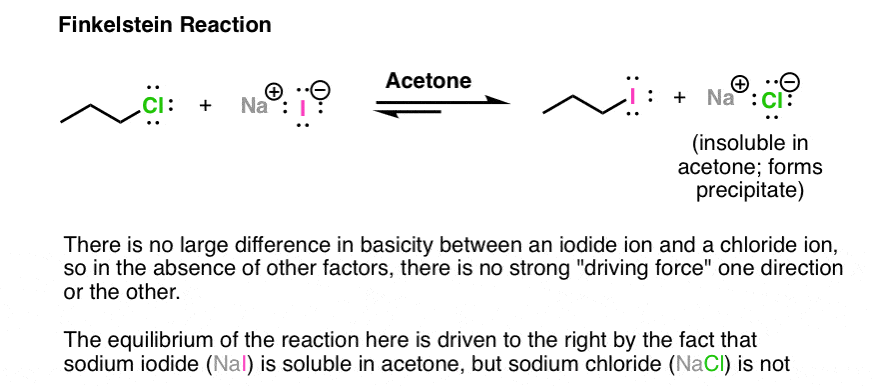
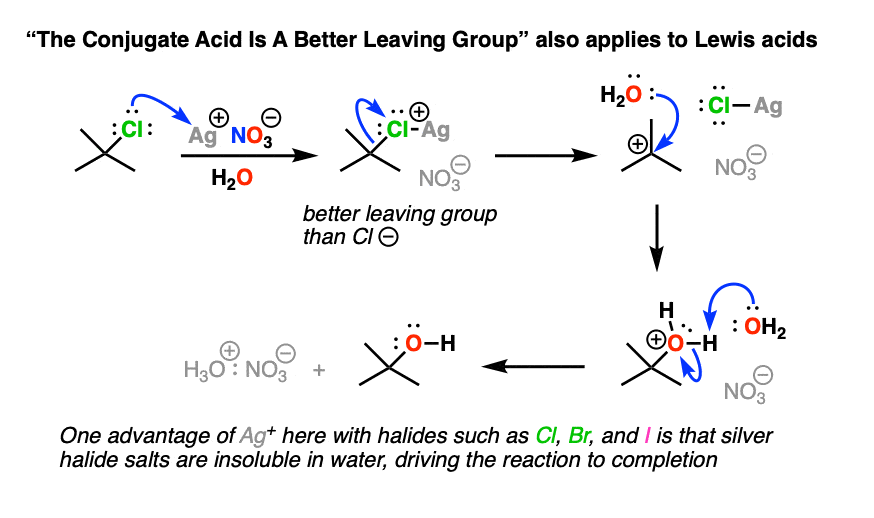
Note 2 – Lewis acids may also be used. With alkyl halides, sometimes silver salts such as AgNO3 or AgBF4 are used to assist with the loss of a Cl or Br group (the counter-ion NO3(-) or BF4(-)is just a spectator here). The Ag(+) coordinates with the Cl or Br, resulting in a much better leaving group (the silver halide salt).
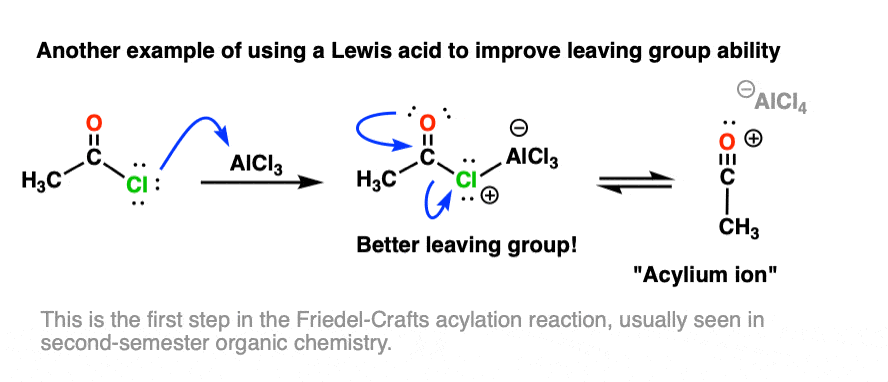
Other examples of Lewis acids serving to increase leaving-group ability are found in several reactions of aromatic compounds, such as the Friedel-Crafts acylation reaction, where a Lewis acid (e.g. AlCl3) assists in loss of Cl from an acid halide:
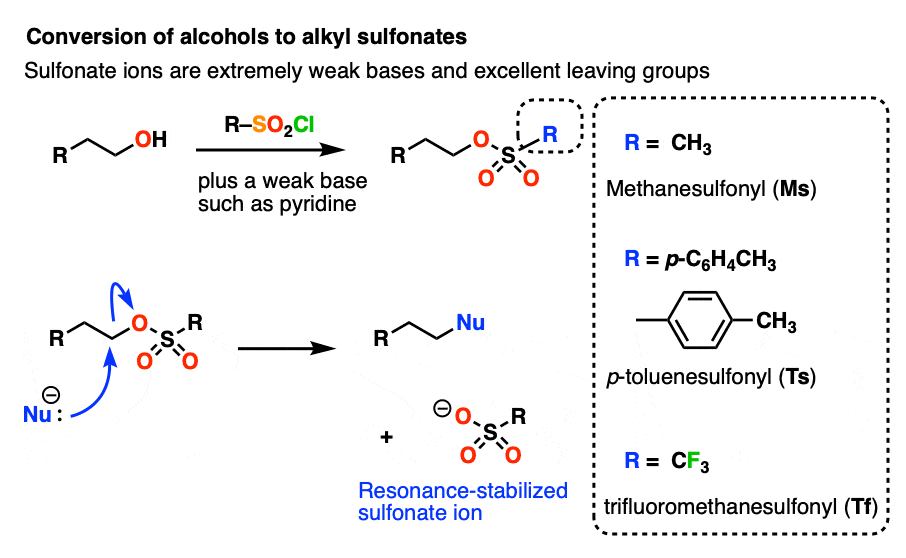
Note 3– Alcohols can be converted into alkyl sulfonates through the use of reagents such as p-toluenesulfonyl chloride (TsCl) or methanesulfonyl chloride (MsCl). Sulfonates are excellent leaving groups since the negative charge on oxygen can be delocalized through resonance. An even better leaving group than (-)OMs or (-)OTs is the trifluoromethylsulfonyl group (-)OSO2CF3 “triflate” group, where the hydrogen atoms on OMs have been replaced with electron-withdrawing fluorines.
Note 4 – Fluorine is a poor leaving group in nucleophilic substitution reactions, but can, in certain cases, be a leaving group in various elimination reactions. One example is the E1cb mechanism, where the first step is formation of a conjugate base, followed by (rate-determining) elimination of a leaving group.
Another example of fluorine as a leaving group is in the nucleophilic aromatic substitution reaction, where the electron-withdrawing F actually helps to make the carbon more electrophilic. Sanger’s reagent, which was used to determine the sequence of peptides in insulin, is a useful example of a reagent for promoting nucleophilic aromatic substitution that has fluoride leaving groups.
Note 5 – It’s possible for a species to be both a good nucleophile and a good leaving group. Nucleophilicity is defined through the rate of reaction of a nucleophile with a given substrate. The carbon-iodine bond is fairly weak, however (about 50 kcal/mol), and readily undergoes cleavage.
Note 6 – A more full pKa table.
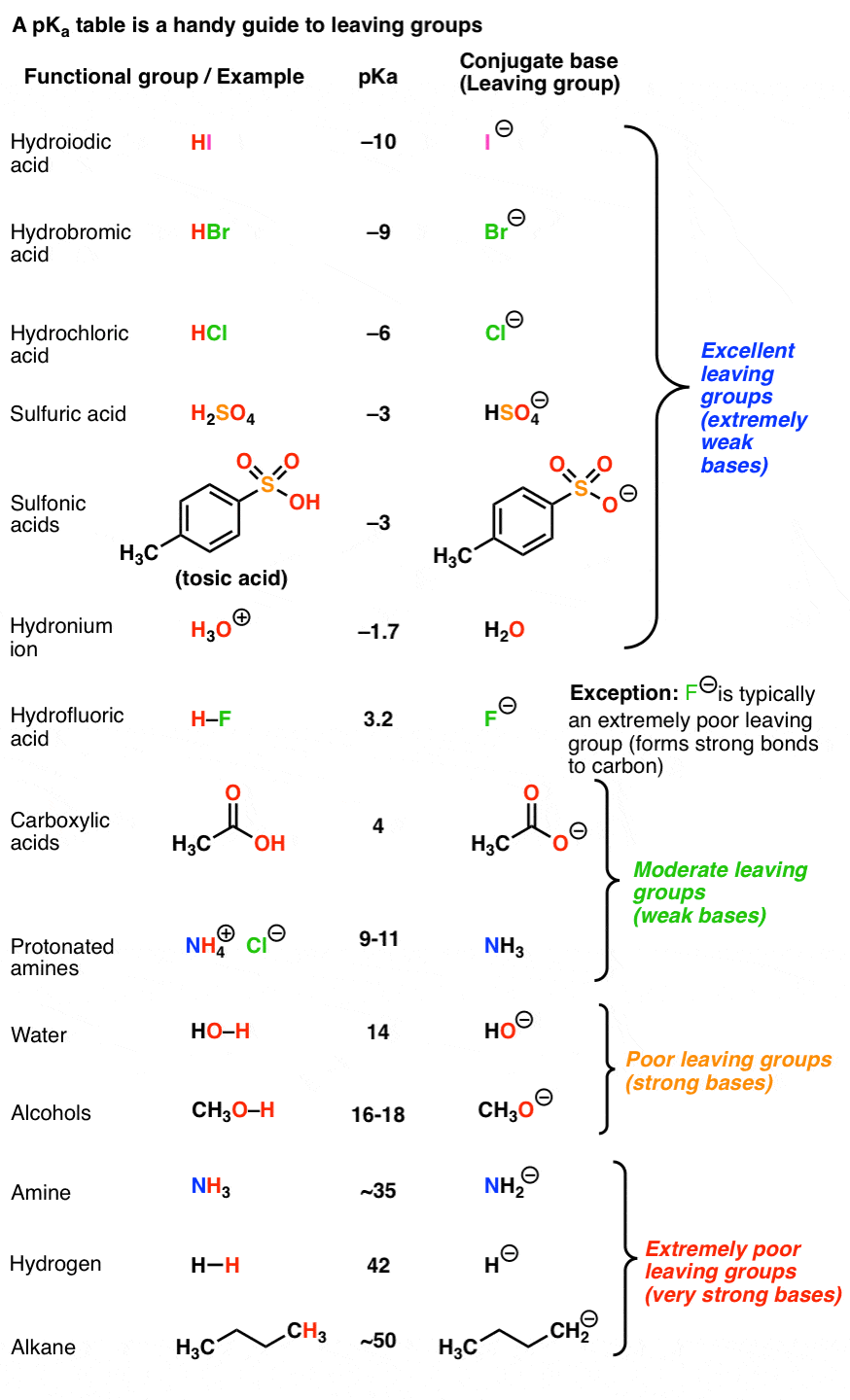
Note 7. My personal vote for “best leaving group of all time” is found in this reaction.
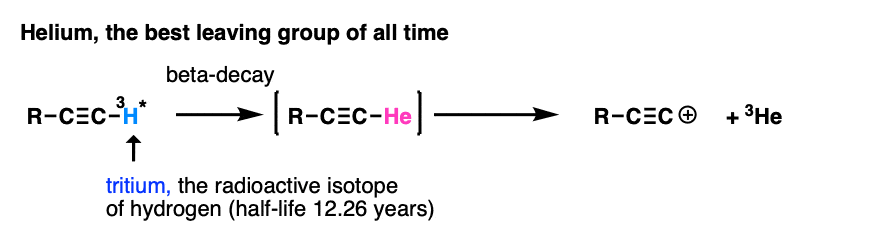
The researchers wanted to study the properties of the alkynyl cation, which is exceptionally unstable and short-lived. However, by putting a radioactive tritium atom on the terminal carbon, a small amount of beta-decay resulted in a helium atom, which promptly left, resulting in an alkynyl cation (which reacted with the solvent, benzene). Of course they didn’t wait around for 12 years to get to 50% completion :-) [Ref – J. Am. Chem. Soc. 1988, 110, 1298 ]
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.

Become a MOC member to see the clickable quiz with answers on the back.
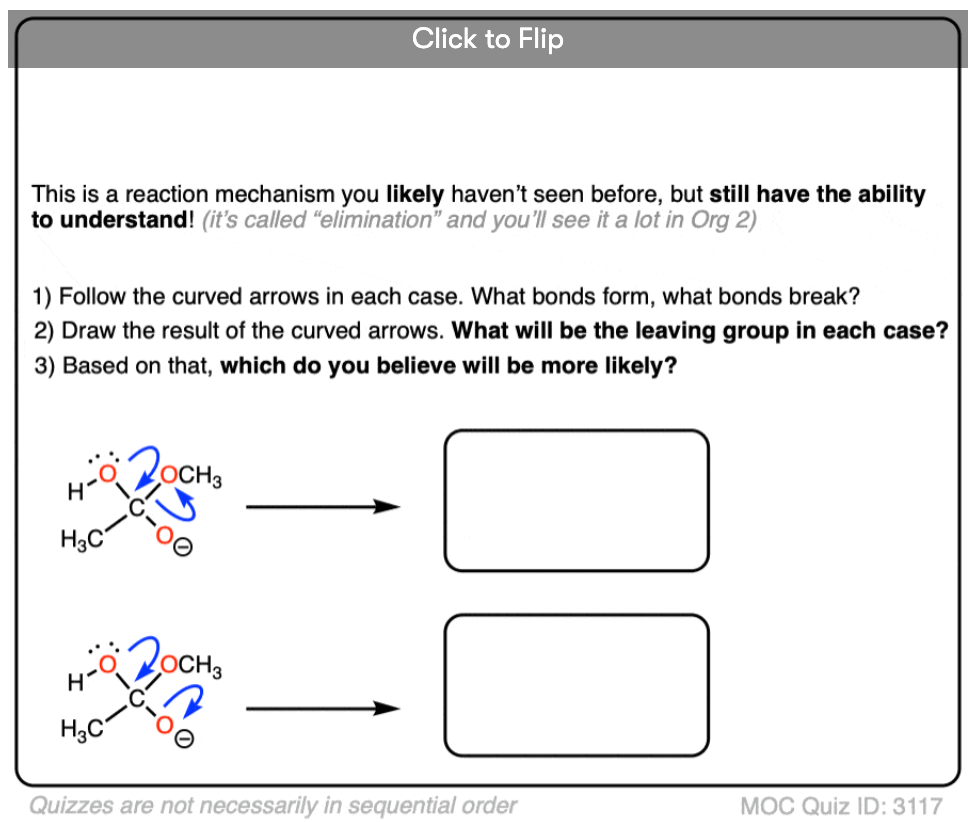
Become a MOC member to see the clickable quiz with answers on the back.
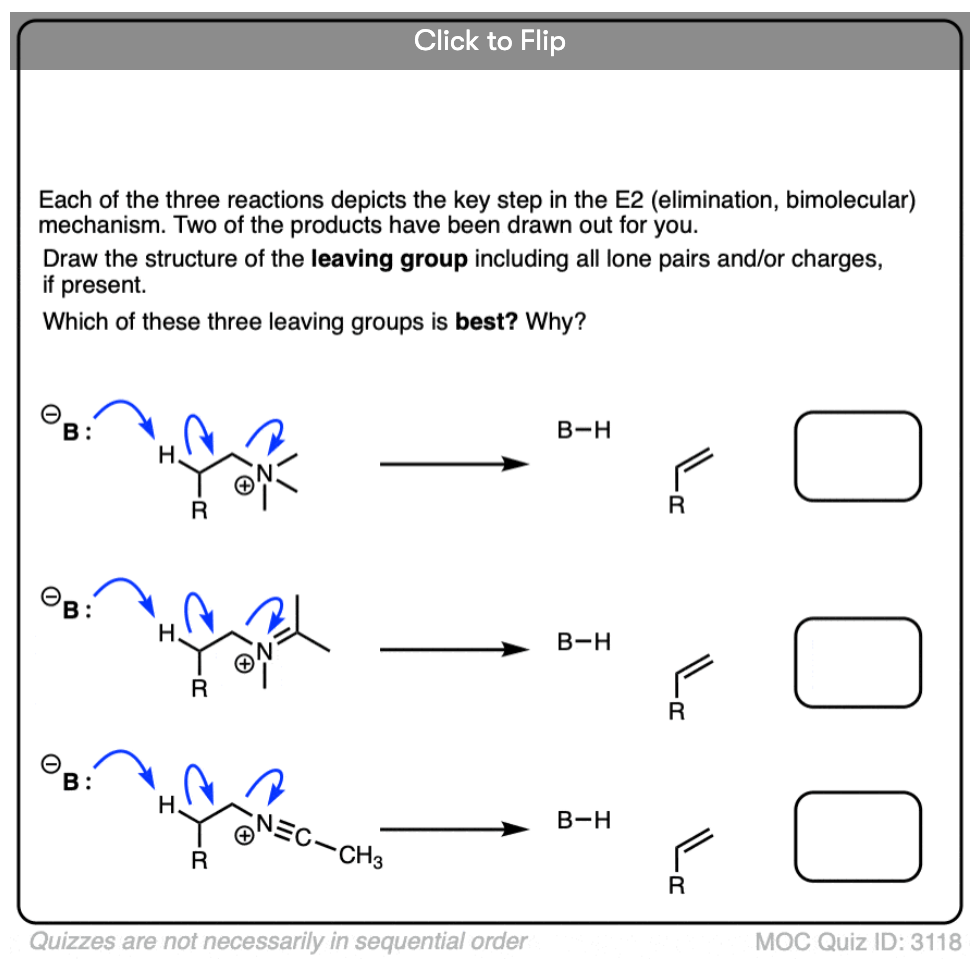
Become a MOC member to see the clickable quiz with answers on the back.
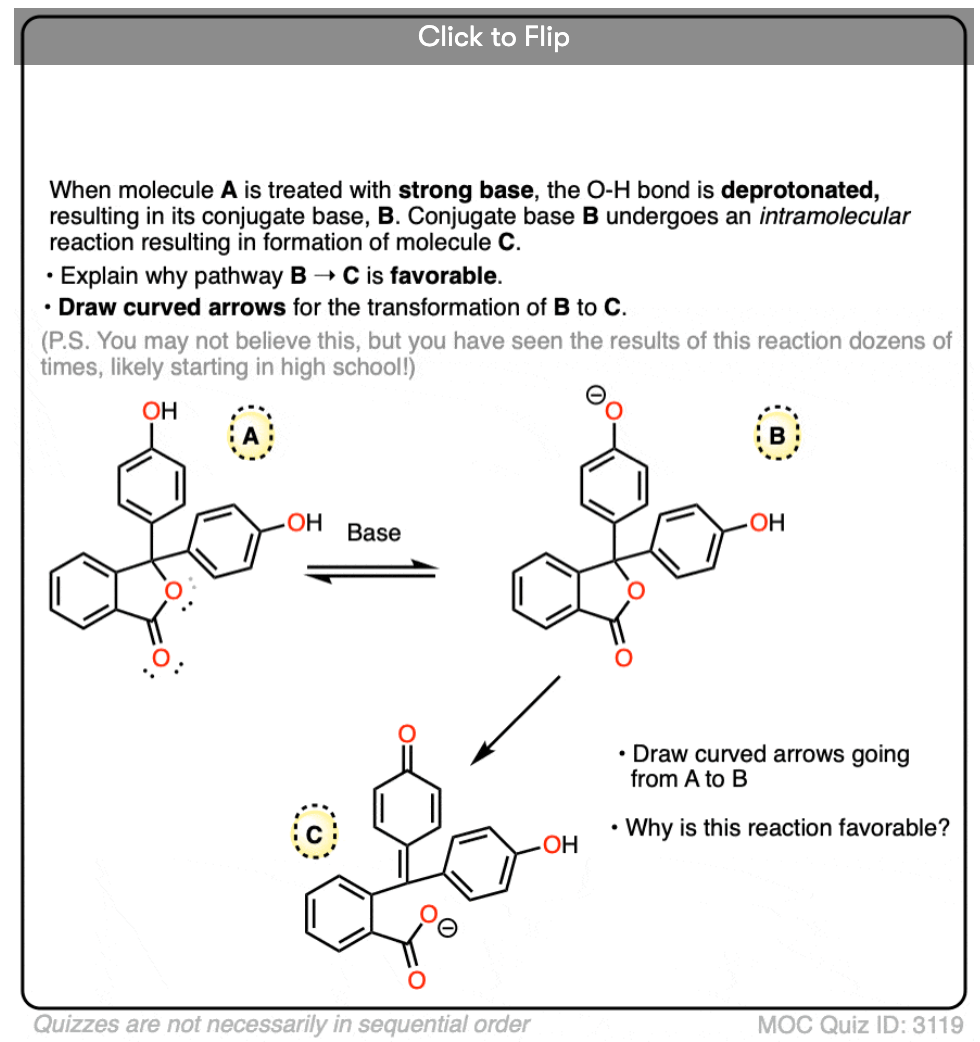
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
- Generation and trapping of an alkynyl cation
Giancarlo. Angelini, Michael. Hanack, Jan. Vermehren, and Maurizio. Speranza
Journal of the American Chemical Society 1988 110 (4), 1298-1299
DOI: 10.1021/ja00212a052 - The Effect of the Carbonyl and Related Groups on the Reactivity of Halides in SN2 Reactions
F. G. Bordwell and W. T. Brannen
Journal of the American Chemical Society 1964 86 (21), 4645-4650
DOI: 10.1021/ja01075a025
That’s awesome but I don’t understand why NO2 is meta director while NH2 is Ortho para director
NO2 groups can accept a pi bond from the aromatic ring, making it more electron poor.
https://www.masterorganicchemistry.com/2017/09/26/activating-and-deactivating-groups-in-electrophilic-aromatic-substitution/
I used to be quite confused about how I- could be the best nucleophile and the best leaving group at the same time XP
thank you for these explanations!
Glad you found it helpful. Thank you
H is the least electronegative element. Search its effective nuclear charge. Does it was do be anionic?
Went through many sources..this was really useful!!. Thanks!!!
Could you please explain why H- is a strong base
I guess it has noble gas configuration so won’t donate it’s electron
Please clarify.
If NH3 is more basic than H2O than how is it a Better leaving group than H2O
thank you. this is very helpful I am struggling in organic chem 1 and this helps alot.
This is abs9lutely amazing. I have been struggling since 2 years not being able to understand this. Thank you. .
Can you tell me more about addition elimination reaction with fluorine?
Yes – check out this on nucleophilic aromatic substitution.
https://www.masterorganicchemistry.com/2018/08/20/nucleophilic-aromatic-substitution-nas/
Thank God I found this website in 2020! You might’ve actually saved my year. Cheers!
if a specie is a good nucleophile, is it necessary that it will be a good leaving group
No. Fluoride ion is a good nucleophile, but is not a good leaving group.
This might or might not be related to LG but could you please answer:
Reaction of chloroform with alc. KOH and p-toluidine forms?
Likely…. https://en.wikipedia.org/wiki/Reimer%E2%80%93Tiemann_reaction
Which is better l.g. N2 or H2O?
You would be hard pressed to find a better leaving group than N2.
I’m confused about HF and its conjugate base. Does F- make an excellent leaving group? So for an E2 reaction it would leave?
No, F- makes a terrible leaving group. Part of the reason is that the C-F is extremely strong.
It would definitely not leave in an E2 reaction.
Why are leaving group weak bases, any explanation for this rule??
Think about what’s happening in a reaction where a leaving group is created. You need to break a bond and to form a relatively stable species. So if the bond is too strong (e.g. C-F) or if the leaving group is not very stable by itself (stongly basic) then the activation energy for this process will be too high for it to happen.
in which solvent is this pka table given.
Q. does the leaving tendency depend upon solvent for example if F- leaves in polar protic medium it would stabilize its charge through hydrogen bonding which might not be possible in polar aprotic medium
The C-F bond is very strong, and it’s unlikely to be a leaving group in substitution or elimination regardless of solvent.
Are neutral groups better leaving groups than negatively charged ?
“The conjugate acid is always a better leaving group”. So H2O is a better leaving group than HO-, HOR is better than RO- , NH3 better than NH2 (-). It’s hard to generalize if you combine multiple factors. For instance Cl- is a better leaving group than NH3.
That’s why it’s useful to have a pKa table.
the best explanations can be found here…thank u so much
OK, thank you ankit!
Awesome website, explains the any topic with ease. Whenever I have any doubt I refer MOC, it helps me a lot. Thank you Mr. James for your remarkable efforts. You have made learning organic chemistry interesting through this website.
Do leaving group depend on solvent effect?
Oh. My. Goodness. This is absolutely amazing. I will never be able to thank you enough or express how much this has helped me. Seriously, you are a god in my eyes right now.
Could you please explain the fluoride ion exception?
+NR3 is the best leaving group.
These articles really help. Continue the good work. Cheers from India !
Thank you so much!
why iodine is a good leaving group as well as a good nucleophile
I- is a BLG than F- in aprotic solvents.
F- is a BLG than I- in protic solvents .
If something is a poor nucleophile it has to be BLG.
I am right , right ?
asking this because I read poor bases are BLG which I accept ,but I think that it should differ in solvents.
(Ex:F- in protic is much more stable than I- due h bond.)
someone please tell me if I am right or not.
F-
this is a great website which is helping for my exams very much clarifies my doubts in organic chemistry can you tell me why flurine is a exeption and in a carbonyl carbon or an aromatic ring acts as a good leaving group compared to other halogens
Could u explain why F can become a leaving group when it is attached to a carbonyl carbon or an aromatic ring?
Yes, F- is an OK leaving group in those situations (nucleophilic aromatic substitution) because the rate limiting step is attack of the aromatic ring (disrupting aromaticity). Loss of the leaving group is slow compared to that step. Also, because F- is so electron withdrawing, F actually helps to accelerate the reaction by removing electron density from the ring and making it easier to attack.
James, someone mentioned the difference for F- between protic and aprotic solvents, do you mind shedding some light and clarity on this, please?
Thank you in advance
Question was answered in a round about encompassing way here: https://forums.studentdoctor.net/threads/nucleophiles.752905/
“In polar protic solvents, the nucleophile, which is typically an anion, is strongly solvated by the hydrogen bonding solvent. While you do want the entire reaction to occur in one solution, you don’t want such a strong solvation, because that blocks the anion from reacting quickly with the electrophile. This effect is biggest for fluorides because they are so small and not easily polarisable. In a polar aprotic solvent, this anion solvation doesn’t occur to the same extent, and thus fluoride, which is more reactive than the iodide (think basicity), will be a stronger nucleophile.”
Good read if anyone needs it.
No matter the solvent, F will be a poor leaving group in nucleophilic substitution and elimination reactions.
Minor fix needed on the first image: the reactants side is neutral while the products side is positive (need to either add a negative formal charge to Nu or take off the negative formal charge from L).
Thank you so much!
I ve seen alkoxides getting removed during sn2th reactions (related to carbonyls) . I’m in a mess and get choked up in such reactions.please help
What if there are two identical leaving groups on different parts of the molecule? Say, two bromines. What factors do you look at when choosing the leaving group then?
Then you would examine the carbon that the leaving groups are attached to. Primary, secondary, or tertiary? Then you would look at the reaction conditions (i.e. what nucleophile is present?). This then leads into the SN1/SN2/E1/E2 decision. You might want to check my “Quick N’ Dirty Guide to SN1/SN2/E1/E2 for more information there because it’s a lot to summarize in a blog comment.
By far the most coherent explanation of nucleophiles and leaving groups I have seen so far. Better than Youtube and most text books for that matter!
the site is excellent, thank you very much, you had solved most of my problems in organic chemistry.
Where would O-Ph rank as a leaving group? Is it better than the halides?
Thanks! But can u explain plz why weak bases are better leaving groups?
The more stable the leaving group the better it’s ability to leave right? So, if the base is weak, it will not be in a hurry to accept protons and react. Hence, as the base is not readily reacting, it is stable and a good leaving group.
Weak bases are very stable as anions in solution on their own. A weak base has a strong conjugate acid (e.g. HCl is the strong conjugate acid of the weak base Cl-). In the acid, you can think of the base as being a “leaving group” that allows the H+ to go off on it’s own (Like Cl- rapidly leaving H+ in aqueous solution). Since the acid is strong, we know that the base must very readily leave.
Conversely, if the acid is weak (like CH3COOH), we know that it holds the H+ relatively tightly. The base (CH3COO-) is relatively unstable in solution, so it doesn’t want to leave the H+. It’s very similar when that same base is attached to a carbon skeleton. Cl will leave the carbon skeleton readily because it’s fairly happy just being Cl- in solution, whereas CH3COO- is not so happy to do so. Moderately strong bases, like NH3 are poor leaving groups, and strong bases like OH- are terrible leaving groups. If you take a very strong base like CHC- (acetylide) it will never leave, so we don’t even consider that a leaving group.
Is CN- a good leaving group
No, it’s quite a poor leaving group
Why?
That’s so helpful! You made very simple. Are there other factors beside PKa? what about the bond strength effect in case of a hydroxy gp attched to a carbon…
Thank James
keep up your good work. your site really helped me and my friends most of the concepts in organic chemistry.
There is a mistake in the second paragraph, I think. An atom can never be neutral and charged at the same time. A neutral atom’s atomic number(number of protons) is the same as the number of electrons. I recommend you to say an atom becomes an ion(negatively charged) to reach the full octect.
No he means it BECOMES charged when the electrons move onto the leaving group. I don’t think there’s a mistake.
This website is so useful! Thank you very much!
Just wanted to say thank you. Wish I had found this website months ago. All the information is so accessible and very well organized. Keep up the tremendous work. You’ve gained a loyal visitor to your website. I’ll be back next semester for organic II as well.
Thanks for this amazing resource to help me through orgo. It’s people like you who need to be in Universities teaching students because anyone can see based off this website you’ve made, that you actually care about the people learning and understanding orgo….thank you.
this is a great website, veyr helpful and much clearer than my text or the professor. thank you.
This is just what I need,Organic chemistry has been a part of chemistry that seemed to be impossible to understand. But with the help of your explanations I see my self getting a straight A,beyond the shadow of doubt. Thanks to you James.
What happens if the leaving group is positive, making the charge on the carbon neutral, like S+(CH3)2?
Ah… well the leaving group would be S(CH3)2 which is a very weak base and an excellent leaving group ! (much like OH2(+)]
Yes, please discuss the extra factors you mentioned regarding F- in a-e rxns.
Thanks so much for always making it clear and interesting!
Awesome blog!!!
I have my MCAT exam in 2 days, (well 1.5 days if you start counting from now), wish I stumbled on your blog 3 months ago! well, still 1.5 x 12 hours left! :)
Glad you’re finding it useful. When you’re done, tell your friends :-)
Thank you so much. Very lucid and informative. Please keep it up.
The most understandable way I have ever seen! Thank you very much, please keep up the good work.
Glad you find it helpful Ivana! thanks
Which is the good living group phenoxy ( PhO) or chloride (Cl)?
What’s a weaker base?
Which is the good leaving group phenoxy (PhO) or Chloride Cl?
Procedure for you figuring this out for yourself: Go to a pKa table and figure out the pKa of Phenol (PhOH) and HCl. Which is a stronger acid? Now: the stronger the acid, the weaker the conjugate base. . So which of these would have the weaker conjugate base? The weaker the base, the better the leaving group.
phenol has a pKa of about 16 and HCl has a pKa of about -5 so “strong acid weak conjugate base” would imply that Cl- is the better leaving group.
anyone knows HCl is a strong acid!! STUPID
hcl is a strong acid and it is the better leaving group
how did you get through general chemistry not knowing that?!
phenol at about 10 pka, HCl at about -7 pka. So HCl is the stronger acid=weaker conj. base = more stable as an anion = better leaving group. Do I have this thought process correct?
you’re too awesome, thnx a million!
this was super clear!
I love your site!!! I have been on this more than my text, increasing my quiz score.
Thank you so much!
Glad you find it useful!
Awesome! This clarifies a lot! Thanks!!
Glad you found it useful!
This is gold !!.. Thanks alot
Thanks!