Home / Cleavage Of Ethers With Acid
Alcohols, Epoxides and Ethers
Cleavage Of Ethers With Acid
Last updated: July 5th, 2025 |
Acidic Cleavage of Ethers Can Proceed Through an SN2 or SN1 Mechanism, Depending On The Structure
- Ethers do not undergo very many reactions.
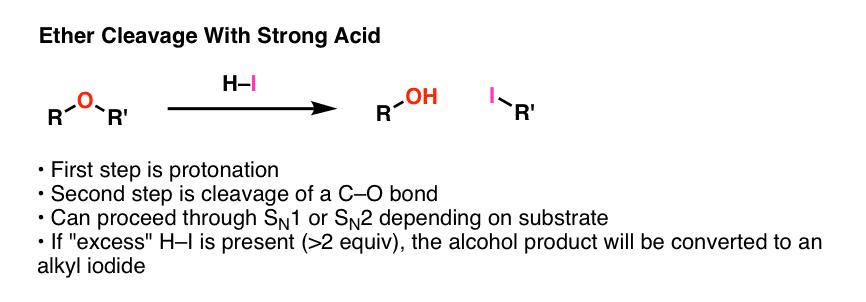
- One key reaction of ethers is that they can undergo cleavage to alcohols in the presence of strong acids, such as HI, or strong Lewis acids such as boron tribromide (BBr3) .
- These reactions involve protonation of the ether oxygen, followed by either an SN1 or SN2 reaction pathway, depending on structure (ethers of primary alcohols tend to undergo cleavage via SN2, ethers of tertiary alcohols tend to undergo cleavage via SN1).
- If excess HI is used for the cleavage of alkyl ethers, the products tend to be alkyl iodides.
- Because ethers are so unreactive to all but the most acidic conditions, they can find use as protecting groups for alcohols.
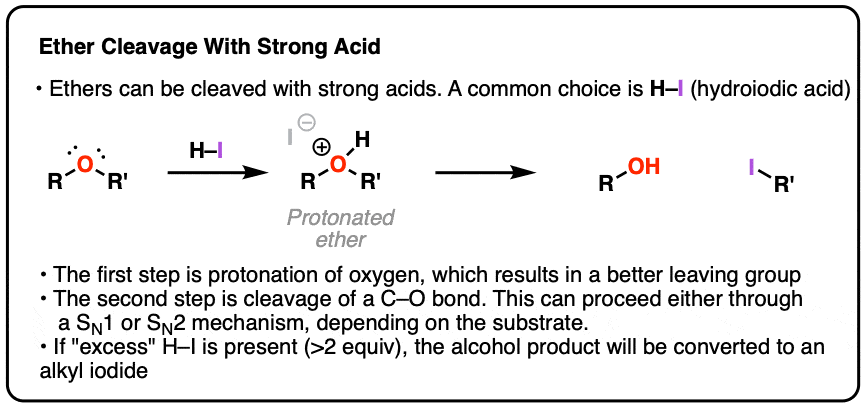
Table of Contents
- All The Reactions Of Ethers In Once Place (har)
- The First Step In Ether Cleavage Is Protonation
- For Methyl and Primary Ethers, The Second Step of Ether Cleavage is SN2
- For Tertiary Ethers, The Second Step of Ether Cleavage is SN1
- For Secondary Ethers, The Second Step Could Occur Through a Mixture of Either Pathway
- The Mechanism for Cleavage of Unsymmetrical Ethers Is Hard To Generalize (With One Exception!)
- Intramolecular Cleavage of Ethers
- Summary: Acidic Cleavage of Ethers
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. All The Reactions of Ethers In One Place (har!)
I’ve been looking forward to today’s post for a long time! We’ve gone through so many different ways of synthesizing ethers, and finally we get to talk about all the exciting things we get to do with them.
Here it is, the moment you’ve been waiting for. All the reactions of ethers in one place:
We have now covered the reactions of ethers.
Thank you for your attention.
Is that it? Yes, really: the only significant reaction of ethers you need to know…. is how to break them.
[I was just pulling your leg about the “exciting things we get to do with ethers” line.]
Does this make ethers the most boring functional group there is? Yes!!! (as long as you don’t count alkanes as a “functional group”).
So, you might ask – what’s the point?
All I’ll say for now is that there are some times when “boring is good”. Ethers, as we’ll learn later, can be useful as “protective groups” for masking (reactive) alcohols. But that’s a later discussion. [See: Protecting Groups For Alcohols]
Right now, let’s dig in to how this ether cleavage reaction works, because it actually does have its subtleties. This discussion should be pretty straightforward if you’ve been following along, however, because it’s just going to involve the familiar mechanisms of protonation, SN1 and SN2.
2. The First Step In Acidic Cleavage Of Ethers Is Protonation Of Oxygen
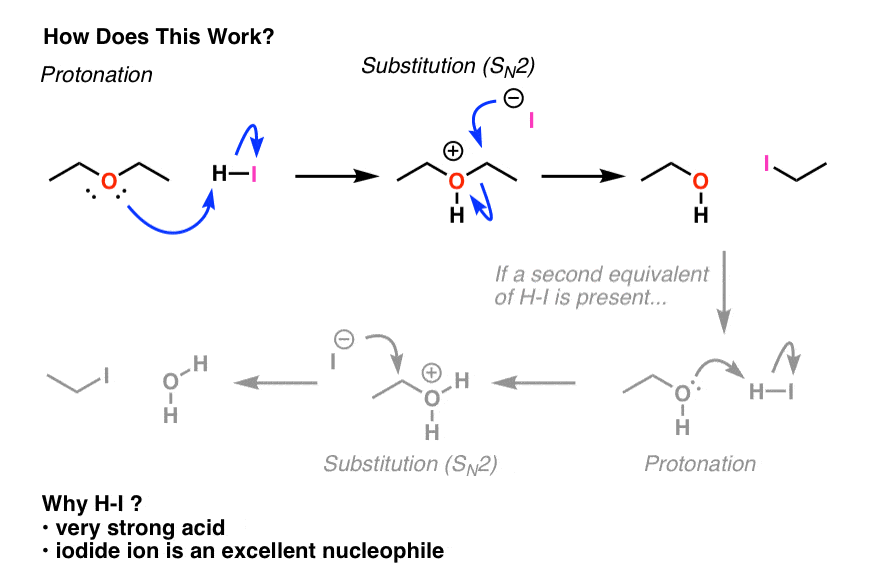
Neutral ethers are generally resistant to nucleophiles in substitution reactions – that’s because the leaving group would have to be RO- , which is a very strong base.
For that reason, the first step in any ether cleavage is protonation by a strong acid. Why does protonation help us? Remember that the “conjugate acid is always a better leaving group” . Protonation of the ether allows for loss of ROH as a leaving group, which is a vastly weaker base than RO- . This is going to set up our next step – cleavage of one of the C–O bonds.
The usual strong acid of choice is usually hydroiodic acid (HI). Not only is it powerful (pKa of –10), as we’ll see the iodide counter-ion plays a role as well.
3. For Methyl And Primary Ethers, The Second Step Of Ether Cleavage Is SN2
After protonation, what happens next? If we start with a primary ether like diethyl ether, we will have a good leaving group (ROH) on a primary carbon in the presence of a decent nucleophile (iodide ion). Sound familiar? It should – these are ideal conditions for an SN2 reaction. And that’s what happens.
The product will be ROH and R-I .
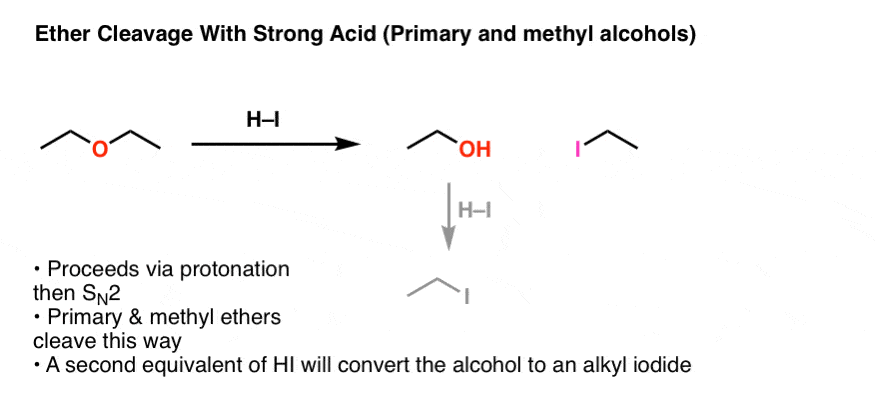
If an excess (2 equiv or more) of HI is present, that alcohol can be converted into an alkyl iodide through two subsequent steps (protonation / SN2).
This “SN2″ pathway will be dominant for primary and methyl ethers.
4. For Tertiary Ethers, The Second Step of Ether Cleavage Is SN1
What about a symmetrical tertiary ether like di-t-butyl ether?
Clearly the SN2 is not in play here, as the tertiary carbons are much too hindered for a backside attack. However, tertiary carbocations are relatively stable – and “ionization” (i.e. loss of a leaving group) leaves us with an alcohol (R-OH) and a tertiary carbocation, which can then be attacked by iodide ion to give R-I
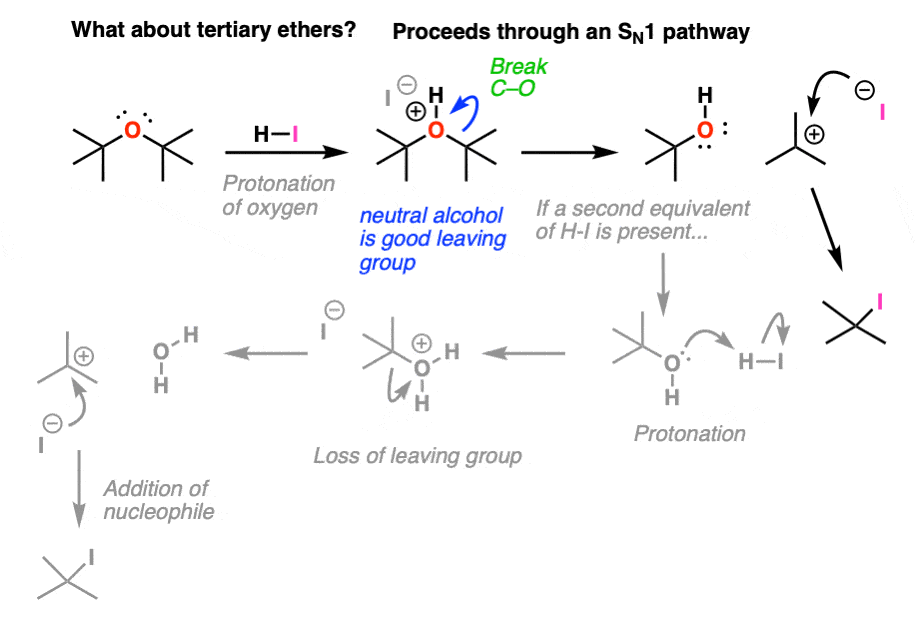
Again, if excess HI is present then that alcohol will be converted into an alkyl halide. We’ll have more about that to say in a few posts actually.
5. For Secondary Ethers, The Second Step Could Proceed Through A Mixture Of Either Pathway
What about secondary ethers? I don’t have a good answer. SN1 and SN2 is a continuum. You’ll likely have a mixture of SN2 and SN1 pathways operating. If someone tells you they can look at an ether like di-isopropyl ether and the SN2 or SN1 pathway will be 100% dominant, that’s just not true.
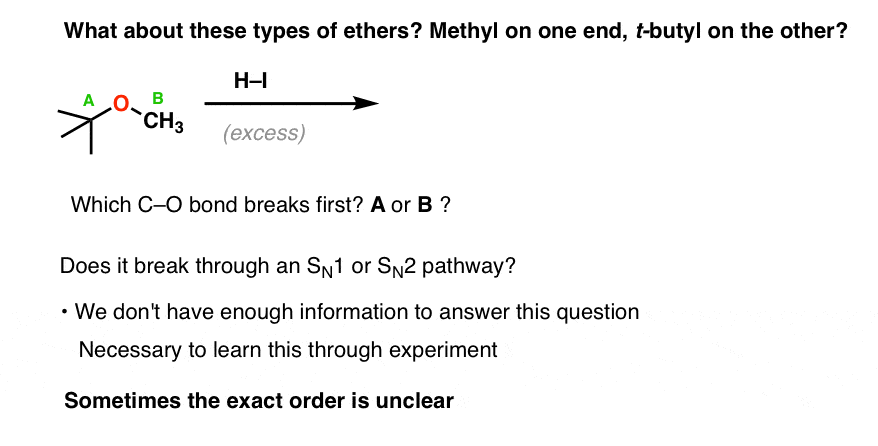
6. The Mechanism For The Cleavage Of Unsymmetrical Ethers Is Hard To Generalize (With One Exception!)
Just as tricky as the case of secondary ethers is the case of “mixed” ethers. What if you have two different groups attached to the oxygen (“unsymmetrical ethers”). Which way is it going to break?
For example, what about t-butyl methyl ether? When you treat it with acid, what happens first? Do you do an SN2 on the methyl group with iodide, or does it ionize to give a tertiary carbocation?
This is the type of question that is NOT easy to answer without knowing the results of experiments.
There are, however, a few cases of mixed ethers where there IS a straightforward answer.
Take this question for example. What happens? The answer is very clear and it goes 100% one way. See if you can do it.
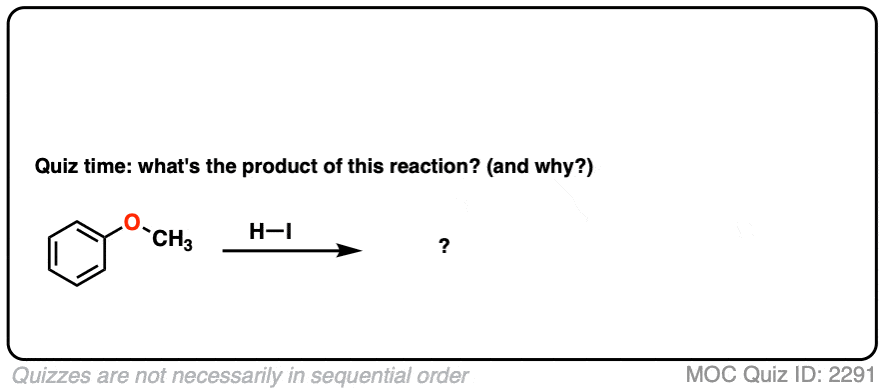 Click to Flip
Click to Flip
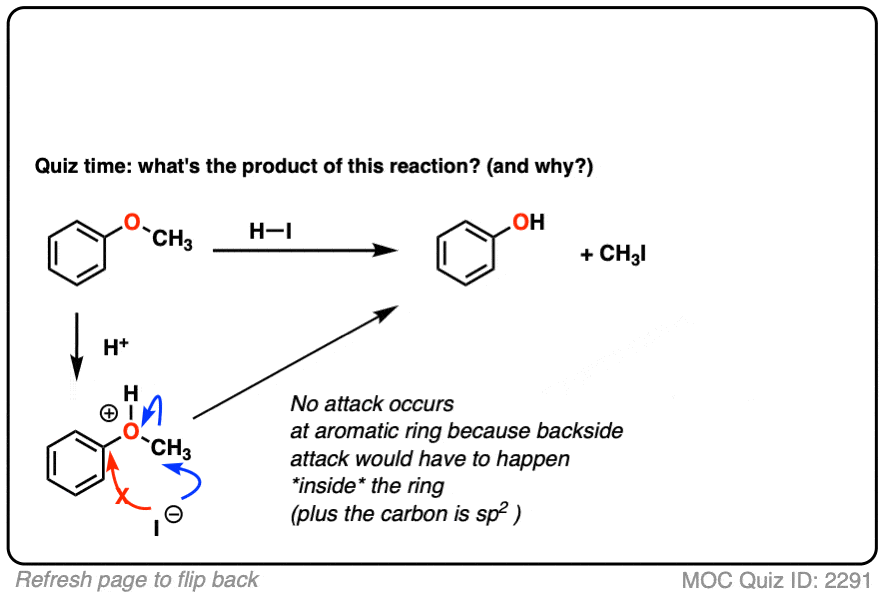
7. Intramolecular Cleavage of Ethers
All right. We’ve seen a few examples of ether cleavage with acid.
Now let’s take tetrahydrofuran (THF), a cyclic ether. And now let’s add strong acid to it. What happens?
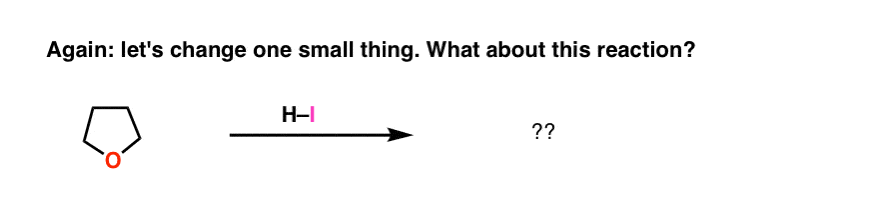
Don’t panic! It’s the same sequence of bonds that form and break as before.
First, we protonate the oxygen to give the conjugate acid, which is now a better leaving group. Second, iodide ion then attacks the carbon, forming C–I and breaking O–C [SN2 in this case].
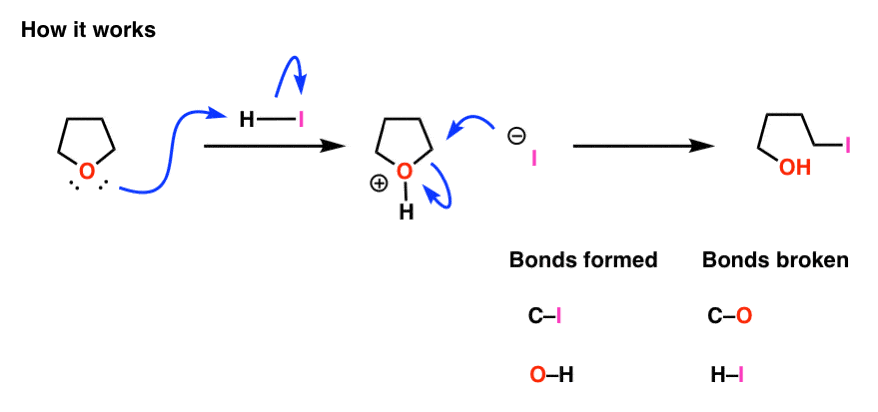
It’s important to note that the bonds that form and break in intramolecular processes (rings forming or breaking) are fundamentally no different than those that form and break in intermolecular processes.
8. Summary: Acidic Cleavage of Ethers
OK. So ethers, as we’ve talked about them so far, ARE pretty boring. But (and there’s always a but) – there IS a special class of ethers which is, in fact, very interesting and very reactive. If you’ve covered alkenes, you’ve seen them before – but under a different name. Can you guess what functional group I’m talking about ? Next post!
Next Post – Epoxides, The Outlier Of The Ether Family
Notes
Related Articles
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
Ethers are widely inert to a lot of conditions, and thus find common use as solvents (e.g. diethyl ether, THF (tetrahydrofuran), dioxane, glyme, and others). Ether cleavage generally requires strong acid and heat, which are forcing conditions. Alternatively, silane reagents can be used, which are reactive at room temperature.
- A NEW METHOD FOR THE PREPARATION OF ORGANIC IODIDES
HERMAN STONE and HAROLD SHECHTER
The Journal of Organic Chemistry 1950, 15 (3), 491-495
DOI: 1021/jo01149a008
Instead of using HI, which is expensive, one can use the combination of phosphoric acid + KI for ether cleavage, which generates HI in situ. - 1,4-DIIODOBUTANE
Herman Stone and Harold Shechter
Org. Synth. 1950, 30, 33
DOI: 10.15227/orgsyn.030.0033
This procedure from Organic Syntheses, a reliable source of independently tested synthetic organic laboratory procedures, demonstrates the cleavage of THF with refluxing strong acid. - The Cleavage of Ethers by Hydrogen Bromide
Robert L. Burwell and Milton E. Fuller
Journal of the American Chemical Society 1957, 79 (9), 2332-2336
DOI: 10.1021/ja01566a085
Classic paper on the cleavage of ethers with HBr.Below are a variety of papers using silane-based reagents for ether cleavage. The Nobel Laureate late Prof. George Olah did a lot of work in this area in the middle of his career. - Cleavage of Esters and Ethers with Iodotrimethylsilane
Tse‐Lok Ho Prof. Dr. George A. Olah
Angew. Chem. Int. Ed. 1976, 15 (12), 774-775
DOI: 10.1002/anie.197607741 - Synthetic methods and reactions. 62. Transformations with chlorotrimethylsilane/sodium iodide, a convenient in situ iodotrimethylsilane reagent
George A. Olah, Subhash C. Narang, B. G. Balaram Gupta, and Ripudaman Malhotra
The Journal of Organic Chemistry 1979, 44 (8), 1247-1251
DOI: 10.1021/jo01322a012 - Trichloro(methyl)silane/Sodium Iodide, A New Regioselective Reagent for the Cleavage of Ethers
George A. Olah, Altaf Husain, B. G. Balaram Gupta, Subhash C. Narang
Angew Chem. Int. Ed. 1981, 20 (8), 690-691
DOI: 10.1002/anie.198106901 - Silane/iodine-based cleavage of esters and ethers under neutral conditions
Ho, T-L., Olah, G. A.
Proc. Natl. Acad. Sci. USA. 1978 Jan; 75 (1):4-6.
DOI: 10.1073/pnas.75.1.4 - Synthetic Methods and Reactions; 32: Mild and Effective Cleavage of Esters and Ethers with Phenyltrimethylsilane/Iodine Reagent
G. A., Ho, T-L.
Synthesis 1977, 417
DOI: 10.1055/s-1977-24423 - Mild cleavage of methoxymethyl (MOM) ethers with trimethylsilyl bromide
Stephen Hanessian, Daniel Delorme, Yves Dufresne
Tetrahedron Lett. 1984, 25 (24), 2515-2518
DOI: 10.1016/S0040-4039(01)81219-3
MOM ethers are commonly used as protecting groups for -OH in organic synthesis, and so strategies for selective deprotection of MOM ethers under mild cleavage are invaluable. - Synthetic Methods and Reactions; 951. Ceric Ammonium Nitrate-Catalyzed Oxidative Cleavage of Alkyl and Silyl Ethers with Sodium Bromate
George A. Olah, B. G. Balaram Gupta, Alexander P. Fung
Synthesis 1980; 1980 (11): 897-898
DOI: 10.1055/s-1980-29258
This does not use silane reagents, but is still an interesting reagent for ether cleavage.
00 General Chemistry Review
01 Bonding, Structure, and Resonance
- How Do We Know Methane (CH4) Is Tetrahedral?
- Hybrid Orbitals and Hybridization
- How To Determine Hybridization: A Shortcut
- Orbital Hybridization And Bond Strengths
- Sigma bonds come in six varieties: Pi bonds come in one
- A Key Skill: How to Calculate Formal Charge
- The Four Intermolecular Forces and How They Affect Boiling Points
- 3 Trends That Affect Boiling Points
- How To Use Electronegativity To Determine Electron Density (and why NOT to trust formal charge)
- Introduction to Resonance
- How To Use Curved Arrows To Interchange Resonance Forms
- Evaluating Resonance Forms (1) - The Rule of Least Charges
- How To Find The Best Resonance Structure By Applying Electronegativity
- Evaluating Resonance Structures With Negative Charges
- Evaluating Resonance Structures With Positive Charge
- Exploring Resonance: Pi-Donation
- Exploring Resonance: Pi-acceptors
- In Summary: Evaluating Resonance Structures
- Drawing Resonance Structures: 3 Common Mistakes To Avoid
- How to apply electronegativity and resonance to understand reactivity
- Bond Hybridization Practice
- Structure and Bonding Practice Quizzes
- Resonance Structures Practice
02 Acid Base Reactions
- Introduction to Acid-Base Reactions
- Acid Base Reactions In Organic Chemistry
- The Stronger The Acid, The Weaker The Conjugate Base
- Walkthrough of Acid-Base Reactions (3) - Acidity Trends
- Five Key Factors That Influence Acidity
- Acid-Base Reactions: Introducing Ka and pKa
- How to Use a pKa Table
- The pKa Table Is Your Friend
- A Handy Rule of Thumb for Acid-Base Reactions
- Acid Base Reactions Are Fast
- pKa Values Span 60 Orders Of Magnitude
- How Protonation and Deprotonation Affect Reactivity
- Acid Base Practice Problems
03 Alkanes and Nomenclature
- Meet the (Most Important) Functional Groups
- Condensed Formulas: Deciphering What the Brackets Mean
- Hidden Hydrogens, Hidden Lone Pairs, Hidden Counterions
- Don't Be Futyl, Learn The Butyls
- Primary, Secondary, Tertiary, Quaternary In Organic Chemistry
- Branching, and Its Affect On Melting and Boiling Points
- The Many, Many Ways of Drawing Butane
- Wedge And Dash Convention For Tetrahedral Carbon
- Common Mistakes in Organic Chemistry: Pentavalent Carbon
- Table of Functional Group Priorities for Nomenclature
- Summary Sheet - Alkane Nomenclature
- Organic Chemistry IUPAC Nomenclature Demystified With A Simple Puzzle Piece Approach
- Boiling Point Quizzes
- Organic Chemistry Nomenclature Quizzes
04 Conformations and Cycloalkanes
- Staggered vs Eclipsed Conformations of Ethane
- Conformational Isomers of Propane
- Newman Projection of Butane (and Gauche Conformation)
- Introduction to Cycloalkanes
- Geometric Isomers In Small Rings: Cis And Trans Cycloalkanes
- Calculation of Ring Strain In Cycloalkanes
- Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane
- Cyclohexane Conformations
- Cyclohexane Chair Conformation: An Aerial Tour
- How To Draw The Cyclohexane Chair Conformation
- The Cyclohexane Chair Flip
- The Cyclohexane Chair Flip - Energy Diagram
- Substituted Cyclohexanes - Axial vs Equatorial
- Ranking The Bulkiness Of Substituents On Cyclohexanes: "A-Values"
- Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
- Fused Rings - Cis-Decalin and Trans-Decalin
- Naming Bicyclic Compounds - Fused, Bridged, and Spiro
- Bredt's Rule (And Summary of Cycloalkanes)
- Newman Projection Practice
- Cycloalkanes Practice Problems
05 A Primer On Organic Reactions
- The Most Important Question To Ask When Learning a New Reaction
- Curved Arrows (for reactions)
- Nucleophiles and Electrophiles
- The Three Classes of Nucleophiles
- Nucleophilicity vs. Basicity
- What Makes A Good Nucleophile?
- What Makes A Good Leaving Group?
- 3 Factors That Stabilize Carbocations
- Equilibrium and Energy Relationships
- 7 Factors that stabilize negative charge in organic chemistry
- 7 Factors That Stabilize Positive Charge in Organic Chemistry
- What's a Transition State?
- Hammond's Postulate
- Learning Organic Chemistry Reactions: A Checklist (PDF)
- Introduction to Oxidative Cleavage Reactions
06 Free Radical Reactions
- Bond Dissociation Energies = Homolytic Cleavage
- Free Radical Reactions
- 3 Factors That Stabilize Free Radicals
- What Factors Destabilize Free Radicals?
- Bond Strengths And Radical Stability
- Free Radical Initiation: Why Is "Light" Or "Heat" Required?
- Initiation, Propagation, Termination
- Monochlorination Products Of Propane, Pentane, And Other Alkanes
- Selectivity In Free Radical Reactions
- Selectivity in Free Radical Reactions: Bromination vs. Chlorination
- Halogenation At Tiffany's
- Allylic Bromination
- Bonus Topic: Allylic Rearrangements
- In Summary: Free Radicals
- Synthesis (2) - Reactions of Alkanes
- Free Radicals Practice Quizzes
07 Stereochemistry and Chirality
- Types of Isomers: Constitutional Isomers, Stereoisomers, Enantiomers, and Diastereomers
- How To Draw The Enantiomer Of A Chiral Molecule
- How To Draw A Bond Rotation
- Introduction to Assigning (R) and (S): The Cahn-Ingold-Prelog Rules
- Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) - The Method of Dots
- Enantiomers vs Diastereomers vs The Same? Two Methods For Solving Problems
- Assigning R/S To Newman Projections (And Converting Newman To Line Diagrams)
- How To Determine R and S Configurations On A Fischer Projection
- The Meso Trap
- Optical Rotation, Optical Activity, and Specific Rotation
- Optical Purity and Enantiomeric Excess
- What's a Racemic Mixture?
- Chiral Allenes And Chiral Axes
- Stereochemistry Practice Problems and Quizzes
08 Substitution Reactions
- Nucleophilic Substitution Reactions - Introduction
- Two Types of Nucleophilic Substitution Reactions
- The SN2 Mechanism
- Why the SN2 Reaction Is Powerful
- The SN1 Mechanism
- The Conjugate Acid Is A Better Leaving Group
- Comparing the SN1 and SN2 Reactions
- Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
- Steric Hindrance is Like a Fat Goalie
- Common Blind Spot: Intramolecular Reactions
- Substitution Practice - SN1
- Substitution Practice - SN2
09 Elimination Reactions
- Elimination Reactions (1): Introduction And The Key Pattern
- Elimination Reactions (2): The Zaitsev Rule
- Elimination Reactions Are Favored By Heat
- Two Elimination Reaction Patterns
- The E1 Reaction
- The E2 Mechanism
- E1 vs E2: Comparing the E1 and E2 Reactions
- Antiperiplanar Relationships: The E2 Reaction and Cyclohexane Rings
- Bulky Bases in Elimination Reactions
- Comparing the E1 vs SN1 Reactions
- Elimination (E1) Reactions With Rearrangements
- E1cB - Elimination (Unimolecular) Conjugate Base
- Elimination (E1) Practice Problems And Solutions
- Elimination (E2) Practice Problems and Solutions
10 Rearrangements
11 SN1/SN2/E1/E2 Decision
- Identifying Where Substitution and Elimination Reactions Happen
- Deciding SN1/SN2/E1/E2 (1) - The Substrate
- Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
- SN1 vs E1 and SN2 vs E2 : The Temperature
- Deciding SN1/SN2/E1/E2 - The Solvent
- Wrapup: The Key Factors For Determining SN1/SN2/E1/E2
- Alkyl Halide Reaction Map And Summary
- SN1 SN2 E1 E2 Practice Problems
12 Alkene Reactions
- E and Z Notation For Alkenes (+ Cis/Trans)
- Alkene Stability
- Alkene Addition Reactions: "Regioselectivity" and "Stereoselectivity" (Syn/Anti)
- Stereoselective and Stereospecific Reactions
- Hydrohalogenation of Alkenes and Markovnikov's Rule
- Hydration of Alkenes With Aqueous Acid
- Rearrangements in Alkene Addition Reactions
- Halogenation of Alkenes and Halohydrin Formation
- Oxymercuration Demercuration of Alkenes
- Hydroboration Oxidation of Alkenes
- m-CPBA (meta-chloroperoxybenzoic acid)
- OsO4 (Osmium Tetroxide) for Dihydroxylation of Alkenes
- Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes
- Cyclopropanation of Alkenes
- A Fourth Alkene Addition Pattern - Free Radical Addition
- Alkene Reactions: Ozonolysis
- Summary: Three Key Families Of Alkene Reaction Mechanisms
- Synthesis (4) - Alkene Reaction Map, Including Alkyl Halide Reactions
- Alkene Reactions Practice Problems
13 Alkyne Reactions
- Acetylides from Alkynes, And Substitution Reactions of Acetylides
- Partial Reduction of Alkynes With Lindlar's Catalyst
- Partial Reduction of Alkynes With Na/NH3 To Obtain Trans Alkenes
- Alkyne Hydroboration With "R2BH"
- Hydration and Oxymercuration of Alkynes
- Hydrohalogenation of Alkynes
- Alkyne Halogenation: Bromination and Chlorination of Alkynes
- Alkyne Reactions - The "Concerted" Pathway
- Alkenes To Alkynes Via Halogenation And Elimination Reactions
- Alkynes Are A Blank Canvas
- Synthesis (5) - Reactions of Alkynes
- Alkyne Reactions Practice Problems With Answers
14 Alcohols, Epoxides and Ethers
- Alcohols - Nomenclature and Properties
- Alcohols Can Act As Acids Or Bases (And Why It Matters)
- Alcohols - Acidity and Basicity
- The Williamson Ether Synthesis
- Ethers From Alkenes, Tertiary Alkyl Halides and Alkoxymercuration
- Alcohols To Ethers via Acid Catalysis
- Cleavage Of Ethers With Acid
- Epoxides - The Outlier Of The Ether Family
- Opening of Epoxides With Acid
- Epoxide Ring Opening With Base
- Making Alkyl Halides From Alcohols
- Tosylates And Mesylates
- PBr3 and SOCl2
- Elimination Reactions of Alcohols
- Elimination of Alcohols To Alkenes With POCl3
- Alcohol Oxidation: "Strong" and "Weak" Oxidants
- Demystifying The Mechanisms of Alcohol Oxidations
- Protecting Groups For Alcohols
- Thiols And Thioethers
- Calculating the oxidation state of a carbon
- Oxidation and Reduction in Organic Chemistry
- Oxidation Ladders
- SOCl2 Mechanism For Alcohols To Alkyl Halides: SN2 versus SNi
- Alcohol Reactions Roadmap (PDF)
- Alcohol Reaction Practice Problems
- Epoxide Reaction Quizzes
- Oxidation and Reduction Practice Quizzes
15 Organometallics
- What's An Organometallic?
- Formation of Grignard and Organolithium Reagents
- Organometallics Are Strong Bases
- Reactions of Grignard Reagents
- Protecting Groups In Grignard Reactions
- Synthesis Problems Involving Grignard Reagents
- Grignard Reactions And Synthesis (2)
- Organocuprates (Gilman Reagents): How They're Made
- Gilman Reagents (Organocuprates): What They're Used For
- The Heck, Suzuki, and Olefin Metathesis Reactions (And Why They Don't Belong In Most Introductory Organic Chemistry Courses)
- Reaction Map: Reactions of Organometallics
- Grignard Practice Problems
16 Spectroscopy
- Degrees of Unsaturation (or IHD, Index of Hydrogen Deficiency)
- Conjugation And Color (+ How Bleach Works)
- Introduction To UV-Vis Spectroscopy
- UV-Vis Spectroscopy: Absorbance of Carbonyls
- UV-Vis Spectroscopy: Practice Questions
- Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model
- Infrared Spectroscopy: A Quick Primer On Interpreting Spectra
- IR Spectroscopy: 4 Practice Problems
- 1H NMR: How Many Signals?
- Homotopic, Enantiotopic, Diastereotopic
- Diastereotopic Protons in 1H NMR Spectroscopy: Examples
- 13-C NMR - How Many Signals
- Liquid Gold: Pheromones In Doe Urine
- Natural Product Isolation (1) - Extraction
- Natural Product Isolation (2) - Purification Techniques, An Overview
- Structure Determination Case Study: Deer Tarsal Gland Pheromone
17 Dienes and MO Theory
- What To Expect In Organic Chemistry 2
- Are these molecules conjugated?
- Conjugation And Resonance In Organic Chemistry
- Bonding And Antibonding Pi Orbitals
- Molecular Orbitals of The Allyl Cation, Allyl Radical, and Allyl Anion
- Pi Molecular Orbitals of Butadiene
- Reactions of Dienes: 1,2 and 1,4 Addition
- Thermodynamic and Kinetic Products
- More On 1,2 and 1,4 Additions To Dienes
- s-cis and s-trans
- The Diels-Alder Reaction
- Cyclic Dienes and Dienophiles in the Diels-Alder Reaction
- Stereochemistry of the Diels-Alder Reaction
- Exo vs Endo Products In The Diels Alder: How To Tell Them Apart
- HOMO and LUMO In the Diels Alder Reaction
- Why Are Endo vs Exo Products Favored in the Diels-Alder Reaction?
- Diels-Alder Reaction: Kinetic and Thermodynamic Control
- The Retro Diels-Alder Reaction
- The Intramolecular Diels Alder Reaction
- Regiochemistry In The Diels-Alder Reaction
- The Cope and Claisen Rearrangements
- Electrocyclic Reactions
- Electrocyclic Ring Opening And Closure (2) - Six (or Eight) Pi Electrons
- Diels Alder Practice Problems
- Molecular Orbital Theory Practice
18 Aromaticity
- Introduction To Aromaticity
- Rules For Aromaticity
- Huckel's Rule: What Does 4n+2 Mean?
- Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems
- Antiaromatic Compounds and Antiaromaticity
- The Pi Molecular Orbitals of Benzene
- The Pi Molecular Orbitals of Cyclobutadiene
- Frost Circles
- Aromaticity Practice Quizzes
19 Reactions of Aromatic Molecules
- Electrophilic Aromatic Substitution: Introduction
- Activating and Deactivating Groups In Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution - The Mechanism
- Ortho-, Para- and Meta- Directors in Electrophilic Aromatic Substitution
- Understanding Ortho, Para, and Meta Directors
- Why are halogens ortho- para- directors?
- Disubstituted Benzenes: The Strongest Electron-Donor "Wins"
- Electrophilic Aromatic Substitutions (1) - Halogenation of Benzene
- Electrophilic Aromatic Substitutions (2) - Nitration and Sulfonation
- EAS Reactions (3) - Friedel-Crafts Acylation and Friedel-Crafts Alkylation
- Intramolecular Friedel-Crafts Reactions
- Nucleophilic Aromatic Substitution (NAS)
- Nucleophilic Aromatic Substitution (2) - The Benzyne Mechanism
- Reactions on the "Benzylic" Carbon: Bromination And Oxidation
- The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions
- More Reactions on the Aromatic Sidechain: Reduction of Nitro Groups and the Baeyer Villiger
- Aromatic Synthesis (1) - "Order Of Operations"
- Synthesis of Benzene Derivatives (2) - Polarity Reversal
- Aromatic Synthesis (3) - Sulfonyl Blocking Groups
- Birch Reduction
- Synthesis (7): Reaction Map of Benzene and Related Aromatic Compounds
- Aromatic Reactions and Synthesis Practice
- Electrophilic Aromatic Substitution Practice Problems
20 Aldehydes and Ketones
- What's The Alpha Carbon In Carbonyl Compounds?
- Nucleophilic Addition To Carbonyls
- Aldehydes and Ketones: 14 Reactions With The Same Mechanism
- Sodium Borohydride (NaBH4) Reduction of Aldehydes and Ketones
- Grignard Reagents For Addition To Aldehydes and Ketones
- Wittig Reaction
- Hydrates, Hemiacetals, and Acetals
- Imines - Properties, Formation, Reactions, and Mechanisms
- All About Enamines
- Breaking Down Carbonyl Reaction Mechanisms: Reactions of Anionic Nucleophiles (Part 2)
- Aldehydes Ketones Reaction Practice
21 Carboxylic Acid Derivatives
- Nucleophilic Acyl Substitution (With Negatively Charged Nucleophiles)
- Addition-Elimination Mechanisms With Neutral Nucleophiles (Including Acid Catalysis)
- Basic Hydrolysis of Esters - Saponification
- Transesterification
- Proton Transfer
- Fischer Esterification - Carboxylic Acid to Ester Under Acidic Conditions
- Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives
- LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes
- Di-isobutyl Aluminum Hydride (DIBAL) For The Partial Reduction of Esters and Nitriles
- Amide Hydrolysis
- Thionyl Chloride (SOCl2) And Conversion of Carboxylic Acids to Acid Halides
- Diazomethane (CH2N2)
- Carbonyl Chemistry: Learn Six Mechanisms For the Price Of One
- Making Music With Mechanisms (PADPED)
- Carboxylic Acid Derivatives Practice Questions
22 Enols and Enolates
- Keto-Enol Tautomerism
- Enolates - Formation, Stability, and Simple Reactions
- Kinetic Versus Thermodynamic Enolates
- Aldol Addition and Condensation Reactions
- Reactions of Enols - Acid-Catalyzed Aldol, Halogenation, and Mannich Reactions
- Claisen Condensation and Dieckmann Condensation
- Decarboxylation
- The Malonic Ester and Acetoacetic Ester Synthesis
- The Michael Addition Reaction and Conjugate Addition
- The Robinson Annulation
- Haloform Reaction
- The Hell–Volhard–Zelinsky Reaction
- Enols and Enolates Practice Quizzes
23 Amines
- The Amide Functional Group: Properties, Synthesis, and Nomenclature
- Basicity of Amines And pKaH
- 5 Key Basicity Trends of Amines
- The Mesomeric Effect And Aromatic Amines
- Nucleophilicity of Amines
- Alkylation of Amines (Sucks!)
- Reductive Amination
- The Gabriel Synthesis
- Some Reactions of Azides
- The Hofmann Elimination
- The Hofmann and Curtius Rearrangements
- The Cope Elimination
- Protecting Groups for Amines - Carbamates
- The Strecker Synthesis of Amino Acids
- Introduction to Peptide Synthesis
- Reactions of Diazonium Salts: Sandmeyer and Related Reactions
- Amine Practice Questions
24 Carbohydrates
- D and L Notation For Sugars
- Pyranoses and Furanoses: Ring-Chain Tautomerism In Sugars
- What is Mutarotation?
- Reducing Sugars
- The Big Damn Post Of Carbohydrate-Related Chemistry Definitions
- The Haworth Projection
- Converting a Fischer Projection To A Haworth (And Vice Versa)
- Reactions of Sugars: Glycosylation and Protection
- The Ruff Degradation and Kiliani-Fischer Synthesis
- Isoelectric Points of Amino Acids (and How To Calculate Them)
- Carbohydrates Practice
- Amino Acid Quizzes
25 Fun and Miscellaneous
- A Gallery of Some Interesting Molecules From Nature
- Screw Organic Chemistry, I'm Just Going To Write About Cats
- On Cats, Part 1: Conformations and Configurations
- On Cats, Part 2: Cat Line Diagrams
- On Cats, Part 4: Enantiocats
- On Cats, Part 6: Stereocenters
- Organic Chemistry Is Shit
- The Organic Chemistry Behind "The Pill"
- Maybe they should call them, "Formal Wins" ?
- Why Do Organic Chemists Use Kilocalories?
- The Principle of Least Effort
- Organic Chemistry GIFS - Resonance Forms
- Reproducibility In Organic Chemistry
- What Holds The Nucleus Together?
- How Reactions Are Like Music
- Organic Chemistry and the New MCAT
26 Organic Chemistry Tips and Tricks
- Common Mistakes: Formal Charges Can Mislead
- Partial Charges Give Clues About Electron Flow
- Draw The Ugly Version First
- Organic Chemistry Study Tips: Learn the Trends
- The 8 Types of Arrows In Organic Chemistry, Explained
- Top 10 Skills To Master Before An Organic Chemistry 2 Final
- Common Mistakes with Carbonyls: Carboxylic Acids... Are Acids!
- Planning Organic Synthesis With "Reaction Maps"
- Alkene Addition Pattern #1: The "Carbocation Pathway"
- Alkene Addition Pattern #2: The "Three-Membered Ring" Pathway
- Alkene Addition Pattern #3: The "Concerted" Pathway
- Number Your Carbons!
- The 4 Major Classes of Reactions in Org 1
- How (and why) electrons flow
- Grossman's Rule
- Three Exam Tips
- A 3-Step Method For Thinking Through Synthesis Problems
- Putting It Together
- Putting Diels-Alder Products in Perspective
- The Ups and Downs of Cyclohexanes
- The Most Annoying Exceptions in Org 1 (Part 1)
- The Most Annoying Exceptions in Org 1 (Part 2)
- The Marriage May Be Bad, But the Divorce Still Costs Money
- 9 Nomenclature Conventions To Know
- Nucleophile attacks Electrophile
27 Case Studies of Successful O-Chem Students
- Success Stories: How Corina Got The The "Hard" Professor - And Got An A+ Anyway
- How Helena Aced Organic Chemistry
- From a "Drop" To B+ in Org 2 – How A Hard Working Student Turned It Around
- How Serge Aced Organic Chemistry
- Success Stories: How Zach Aced Organic Chemistry 1
- Success Stories: How Kari Went From C– to B+
- How Esther Bounced Back From a "C" To Get A's In Organic Chemistry 1 And 2
- How Tyrell Got The Highest Grade In Her Organic Chemistry Course
- This Is Why Students Use Flashcards
- Success Stories: How Stu Aced Organic Chemistry
- How John Pulled Up His Organic Chemistry Exam Grades
- Success Stories: How Nathan Aced Organic Chemistry (Without It Taking Over His Life)
- How Chris Aced Org 1 and Org 2
- Interview: How Jay Got an A+ In Organic Chemistry
- How to Do Well in Organic Chemistry: One Student's Advice
- "America's Top TA" Shares His Secrets For Teaching O-Chem
- "Organic Chemistry Is Like..." - A Few Metaphors
- How To Do Well In Organic Chemistry: Advice From A Tutor
- Guest post: "I went from being afraid of tests to actually looking forward to them".
If there is a conjugated system ie, (CH3)2-C=CH-O-CH=CH2 how will we decide where should be carbocation, and how will double bond get affected?
Sir can we consider the solvent in the case of bond breaking in unsymmetrical ethers???
To identify sn1 or sn2
It is difficult to predict with unsymmetrical di-alkyl ethers. What often happens is that excess H-I is used such that both alkyl fragments are converted into alkyl iodides.
It is easier with aryl alkyl ethers, which will never result in cleavage of the aryl-O bond
If we take isopropyl methyl ether with HI,then what would be the major product? Please tell🙏
Hard to predict from first principles without doing the experiment.
However, one way to know for sure is to use a large excess of HI, which will give you both isopropyl iodide and methyl iodide.
Phenol + Ch3I
We shall obtain phenol and methyliodide if I’m not wrong.
Correct.
what does the reaction of 1,4-dioxane and 1,3-dioxane in dilute acid?
1,3-dioxane is an acetal, and should cleave in mild acid to give formaldehyde and 1,3-propanediol. 1,4-dioxane is not an acetal (it is an ether) and requires harsh acid to cleave (e.g. HI)
what does the reaction of 1,4 dioxane with excess HI give?
Replace each C-O bond with a C-I bond and you get the idea.
In part 4, “Again, if excess HI is present then that alcohol will be converted into an alcohol.”
it is alkyl halide
Fixed. Thank you!
So why are MOM protecting groups typically removed with HCl?
MOM is not an ether, it’s an acetal. Much easier to cleave. https://www.organic-chemistry.org/protectivegroups/hydroxyl/mom-ethers.htm
can ethers react with hcl?
Generally, HCl will be poorer at cleaving ethers than HI.
What if we proceed hydrolysis of methyl vinyl ether in acidic medium. What I wish to know is should we protonate ether first or hydrolyze alkene??
Thanks for your awesome work
Vinyl ethers are a special case.
The first step is protonation of the alkene, which is unusually reactive due to the presence of pi-donating oxygen. The second step is addition of water, which forms a hemiacetal and is then easily hydrolyzed.
Sir if conc HI is used what would be the change if any?
Any alcohols present would likely be converted to alkyl halides.
Phenol and methyliodide
Is there a difference when aqueous HI and anhydrous HI + ether is used ?
In aqueous HI the strongest acid possible is H3O+, (pka of about -3) whereas in anhydrous HI the strongest acid possible is HI itself (pKa -10). So anhydrous HI is much more powerful.
Answer to the quiz problem is phenol +CH3I
Yes.
Still not too sure how this works
Wouldn’t the methyl/ethyl carbocation react back with the newly formed compound to give back the original compound as they both are excellent bases?
Isn’t iodide a weaker base than water which would make it a better leaving group?
Also, how does iodide being both a good nucleophile (polarizable) and a good leaving group (polarizable) add up? Which take precedence?
In the paragraph under “Case #2 – Tertiary Ethers”, I believe “Again, if excess HI is present then that alcohol will be converted into an alcohol” should read “alcohol will be converted into an alkyl iodide” instead.
Don’t say that this is the one and only possible reaction to an organometallic chemist!
Good article, thanks.
Ha. Well, it’s the one key reaction undergrads learn in introductory courses.
Yeah, sure. To advance that on slightly, you can discuss the mechanism of deprotection of methoxy groups by BBr3, being not far removed from that which you have written about.
True!
The article is great. Thank you for sharing. Hope to hear more from you.
First step is the protonation of the oxygen, leaving a positive charge on the latter.
For the second step there are certainly two possible ways:
– A Sn1 reaction with Phenol or Methanol as leaving group, forming either a rather unstable methyl cation or a very unstable benzene cation, respectivley. Both ways seem to be not as reasonable as the second way…
– A Sn2 reaction. After Protonation of the oxygen, there is a nucleophilic attack of the iodide to the methylcarbon, generating Phenol as leaving group and Methyl-Iodide.
Another possible consideration would be the nucleophilic attack of iodide at the rather electron-rich aromatic carbon connected to the oxygen. That wouldn´t be a reasonable mechanism.
Right on the money!
Why is the benzylic cation unstable? I thought it would be stable because of resonance.
I thought the phenylmethylether would react through an SN1 mechanism so that the phenyl gets the I and the methyl gets the OH
Ah – what you call the “benzylic” carbon is actually the phenyl cation (C6H5+). The p orbital of the carbocation is in the same plane as the hydrogens in the benzene ring – perpendicular to the pi bonds which would be able to stabilize it through resonance.
What we term the “benzylic” carbon is actually C6H5CH2+ . This has a carbocation adjacent to a phenyl ring, which CAN be stabilized by resonance.
I should write a post on this common source of confusion.
Actually, you must since it is a major confusion between people about benzyl and phenyl groups
Yes, it’s just one of those things you have to get used to and move on.
Yeah who decided that “benzene” is C6H6 yet C6H5 is a “phenyl” group and that a “benzyl” group refers to benzene with a methyl attached to the ring? I think it is a source of confusion for many who are new to O-chem.
Yes, absolutely!
I still am unsure why it is assumed in the Sn2 reaction that the methylcarbon (and not the ring) will be attacked by the iodide? Is this something to do simply with the characteristic of the Sn2 reaction that I’m missing? I would think that a carbocation could be formed in either ‘direction’, and therefore the iodide could attack either side.
It’s important to recall the factors affecting carbocation stability that you likely learned during SN1/SN2. Carbocations increase in stability with substitution of carbon, so tertiary carbocations are far more stable than methyl carbocation. The SN2 is sensitive to steric hindrance since the nucleophile must do a backside attack, so methyl (and primary) is much faster than tertiary.
Bottom line is that the methyl carbocation will not form, and an SN2 will dominate and the fastest SN2 will occur at the methyl carbon. An sn2 is not possible on the aromatic ring because the empty sigma-star orbital (where the nucleophile would need to attack) is buried in the middle of the aromatic ring and is therefore inaccessible).
Another informative article. About the question at the end, will HI add to anisole to give Iodobenzene?
Think about the SN2 reaction. It has to go through a backside attack. Is one SN2 more favoured than the other?
One of the products will surely be phenol…cause once phenol is produced, it will not be converted to the iodo compound :)
Phenol is indeed one of the products.
I’d say phenol is formed because a phenyl carbocation or a phenyl carbocation character would be much more unstable than methyl carbo cation. Thus phenol and methyl iodide are formed.
It would not go through a methyl cation, it would go via SN2.