Aromaticity
Antiaromatic Compounds and Antiaromaticity
Last updated: April 23rd, 2025 |
Antiaromatic Compounds Are Unusually Unstable
What are the key factors that determine whether a molecule is antiaromatic?
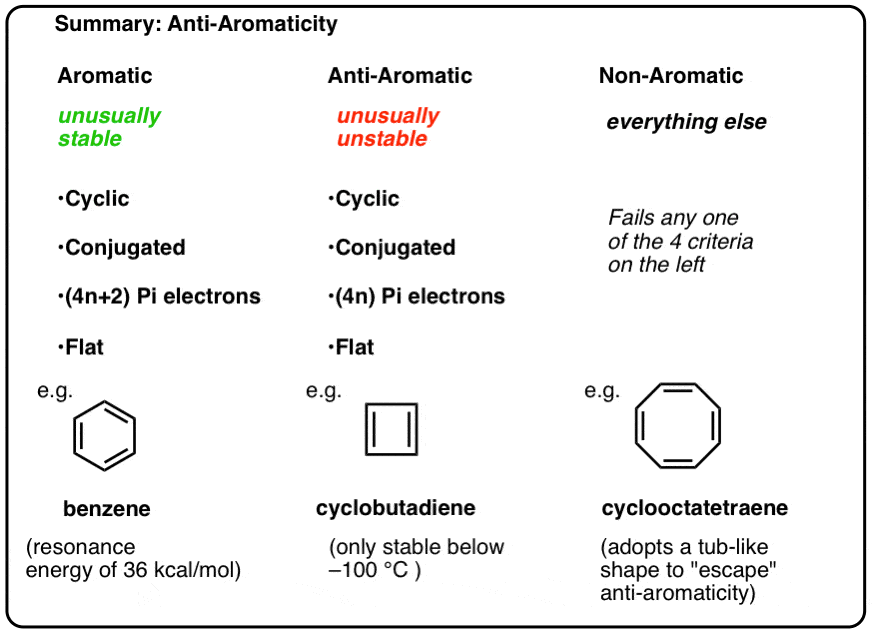
It is similar to the requirements for aromaticity, except for one key factor (in red).
- The molecule must be cyclic
- The molecule must be conjugated all the way around the ring
- The molecule must be flat
- The molecule must have (4, 8, 12, 16…) pi electrons (summarized algebraically as “4n where n is a natural number”). Contrast this with aromatic molecules, which must have (2, 6, 10, 14, 18…) pi electrons.
Anti-aromatic molecules are unusually unstable.
In our previous posts in our series on aromaticity [intro to aromaticity], [rules for aromaticity], we saw that aromatic molecules are unusually stable. They have particularly large resonance energies, tend to undergo substitution rather than addition reactions, and have delocalized pi electrons (all the C-C bond lengths in benzene are equal, for example).
In order to be aromatic, a molecule must possess the following four structural characteristics:
- cyclic
- conjugated all around the ring
- have [4n+2] pi electrons [equivalently: the number of pi electrons must equal twice an odd number]
- flat (planar)
If these four conditions are met, the molecule is aromatic.
If it flunks even one question on the test, it’s non-aromatic.
That seems to be pretty clear. Or is it?
You might guess from the way I’m framing this question that the answer is “not quite”. : – )
Table of Contents
- Some Molecules Are So Spectacularly And Unusually Unstable And Hard-To-Isolate That They Deserve Their Own Category:Antiaromatic
- Cyclopentadienyl Cation: The “Resonance-Stabilized” Carbocation That Isn’t Stable
- Antiaromatic Three-Membered Rings: Oxirene, 1H-Azirene, Thiirene
- Cyclobutadiene Is Antiaromatic
- An 8 Pi-Electron Example of an Antiaromatic Compound: Pentalene
- What Makes A Molecule Anti-Aromatic?
- Cyclooctatetraene “Escapes” Anti-Aromaticity Through Twisting Out Of Flatness
- Conclusion: Antiaromatic Compounds
- Notes
- Quiz Yourself!
- Advanced: References and Further Reading
1. Spectacularly Unstable Molecules That Form A Category Unto Themselves: “Anti-Aromatic”
Here’s the thing. There’s a small number of molecules that flunk the aromaticity test that aren’t just non-aromatic: they have the property of being so spectacularly and unusually unstable and difficult to isolate that they deserve another name.
We call these molecules “anti-aromatic“.
What’s interesting is that in many cases the molecules themselves don’t look to be particularly unstable from first principles, in contrast with, say, cubane. Funny enough, you can buy cubane (more or less) from Aldrich, but most of the anti-aromatic molecules we’ll discuss below are only stable at extremely low temperatures – if they can be isolated at all!
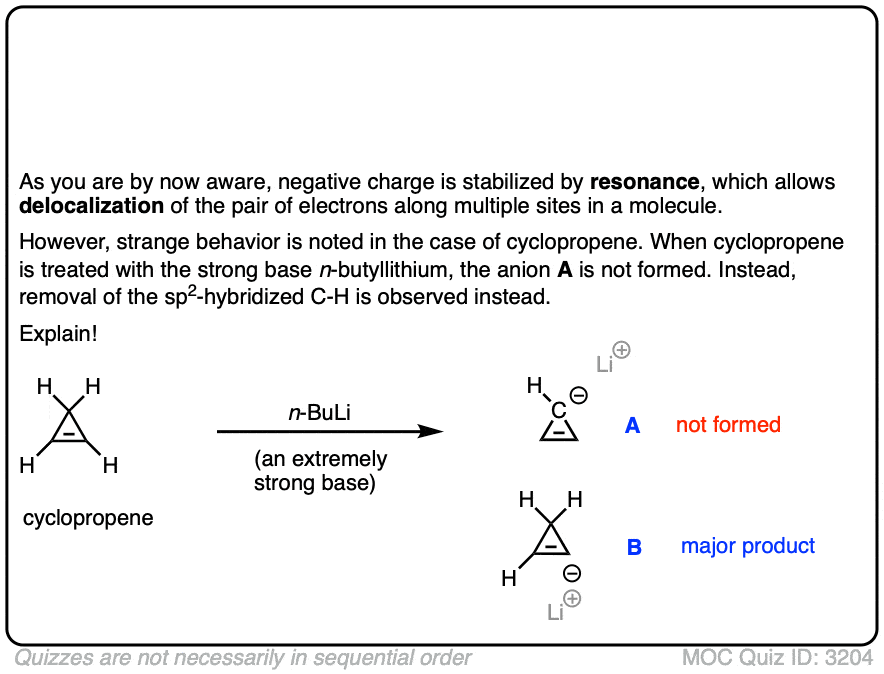
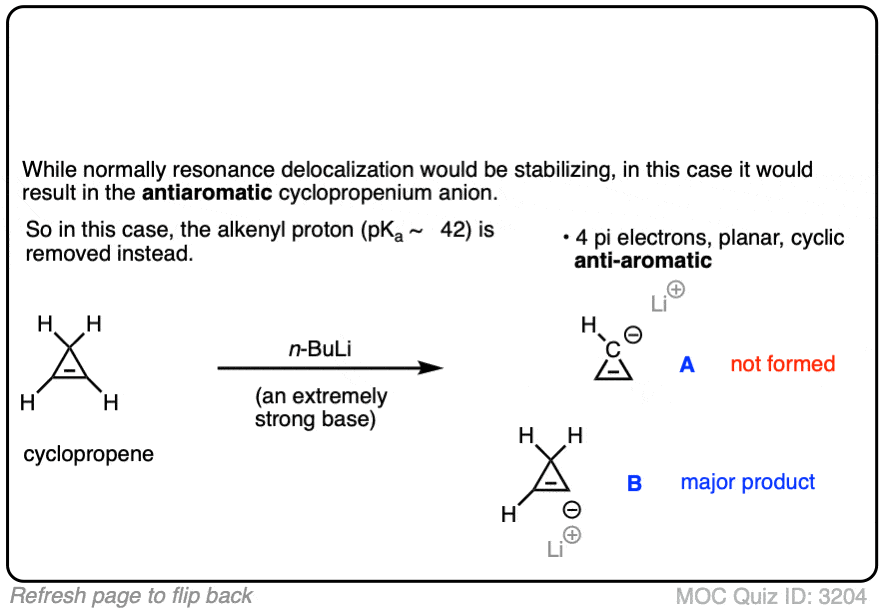
2. Cyclopentadienyl Cation: The “Resonance-Stabilized” Carbocation That Isn’t Stable
Let’s start our journey down the rabbit hole with a simple example that should seem familiar from first-semester organic chemistry.
Remember the SN1 reaction?
The cat that leaves the comfy chair first? (see post: The SN1 Reaction)
Quick review: Start with an alkyl halide. Leaving group leaves, forming a carbocation. Nucleophile attacks. It’s called the SN1 because the rate-determining step (carbocation formation) is unimolecular. Bottom line: the more stable the carbocation, the faster the reaction proceeds. Hence, the reaction rate for alkyl halides is tertiary > secondary > primary, and the carbocation gets special bonus points if it’s stabilized by resonance.
Look at these two SN1 reactions. Which one do you think would happen faster – the SN1 reaction which goes through a secondary carbocation (A), or the SN1 reaction which goes through a secondary carbocation and can form multiple resonance forms (B)?
From everything you’ve learned so far, you’d expect the answer to be B).
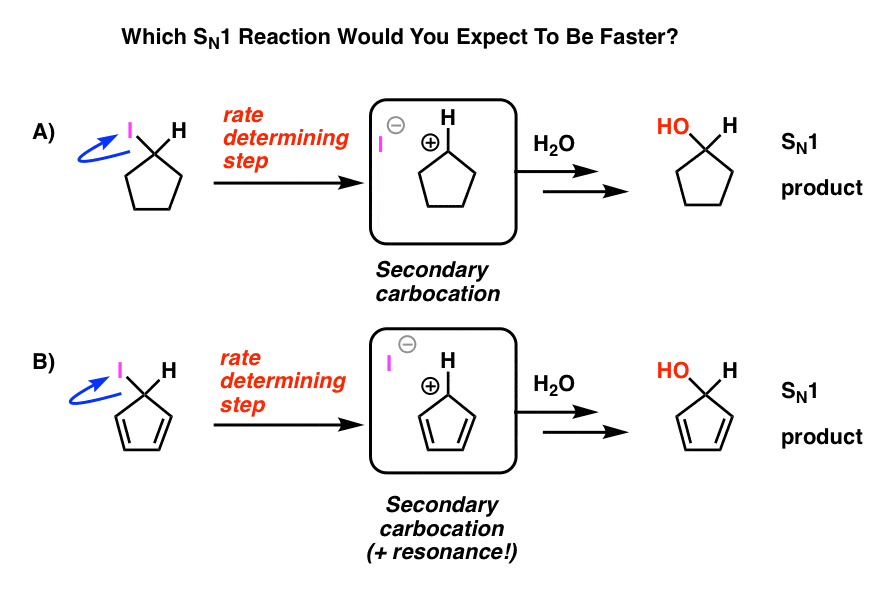
It’s not.
The answer is that reaction A) happens faster. Reaction B does not happen at all. [Note 1]
This should strike you as deeply weird.
It is!
How is it that this supposedly resonance-stabilized carbocation is less stable than a secondary carbocation?
After all, how many times is it drilled into your head that resonance stabilizes molecules, especially carbocations?
But here, it seems to be destabilizing. It’s… it’s…

But unstable it is! The cyclopentadienyl cation is incredibly unstable and difficult to make – and it’s not for lack of trying. There’s something very strange about the structure of the cyclopentadienyl cation that gives it unusual instability.

Cyclic, conjugated, flat…. and has 4 pi electrons. Interesting!
3. Antiaromatic Three-Membered Rings: Oxirene, 1H-Azirene, Thiirene
Another class of “elusive” molecules is a family of three-membered rings. You might recall from Org 1 that it’s easy to make epoxides from alkenes using an oxidant like m-CPBA.
Did you ever wonder why we never covered the same reaction for alkynes?
Well, it’s not for lack of trying. Chemists have tried all kinds of methods for epoxidizing alkynes, and you know what? The reaction just doesn’t frickin’ work.
For instance, epoxidation of acetylene would give the molecule below (oxirene).
Oxirene itself has never been observed, although there are tantalizing traces of its fleeting existence. And by the way, neither has the nitrogen analogue, 1H-azirene, or the thiirene. [Note 2]
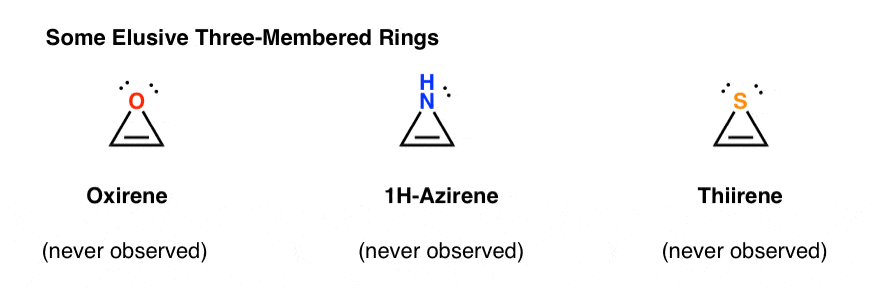
Why not? What’s special about each of these cases?
You might notice that, like the cyclopentadienyl cation, these molecules are cyclic, conjugated, flat, and have 4 pi electrons (two in the pi bond, and two from a lone pair). [Note 3]
OK. What else might be cyclic, conjugated, flat, and have 4 pi electrons?
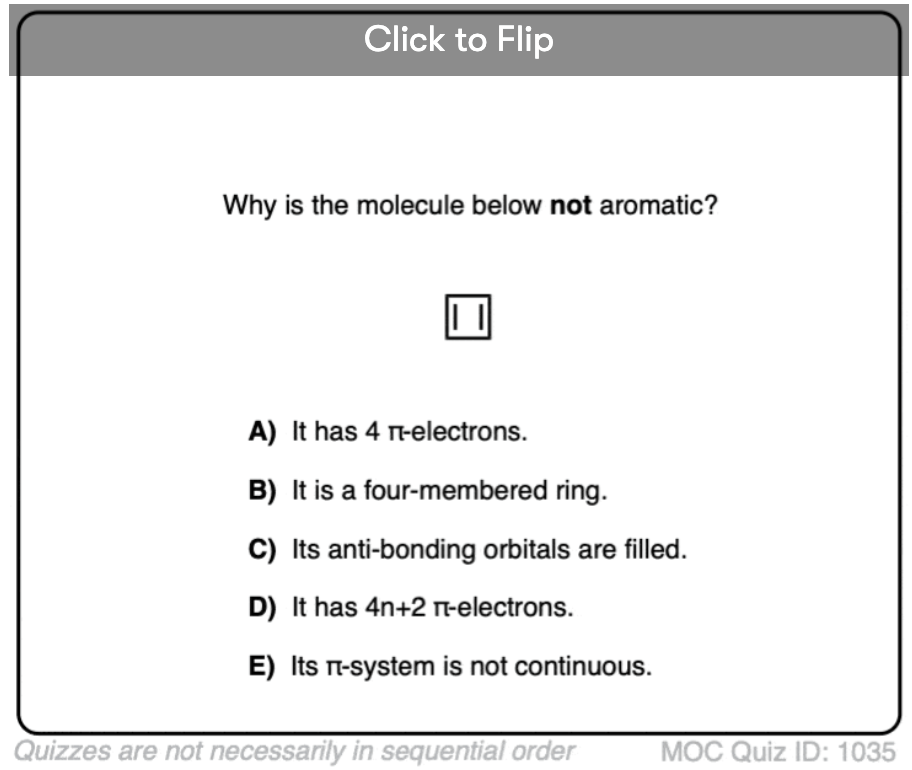
4. Cyclobutadiene Is Antiaromatic
Cyclobutadiene looks like a simple enough molecule, but it wasn’t actually synthesized until 1965. And even then, it was found that it isn’t stable at temperatures above 35 Kelvin. [Note 4]
The question is, why?

Sure, it’s a four-membered ring, and yes, it has a lot of ring strain, but more strained molecules have been made that are actually stable at room temperature.
You might also note that like the above examples, cyclobutadiene is another example of a molecule that is cyclic, conjugated, has 4 pi-electrons, and is flat.
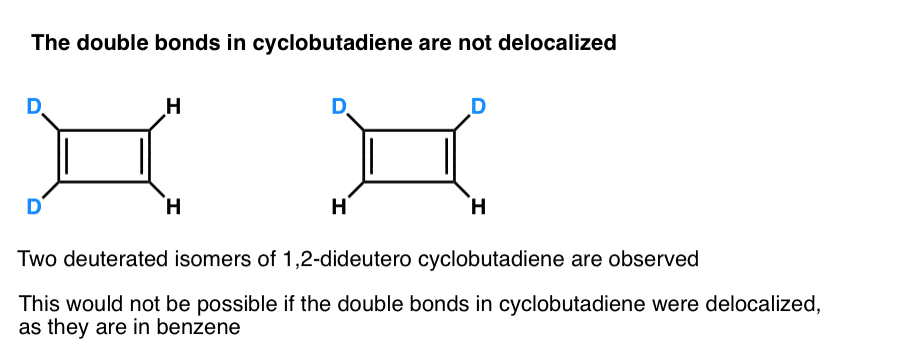
What’s even more interesting is what was learned about the geometry of cyclobutadiene. Rather than being a molecule with identical bond lengths (like benzene), cyclobutadiene was found to have a rectangular shape, indicating that the electrons were not delocalized. [Note 3]
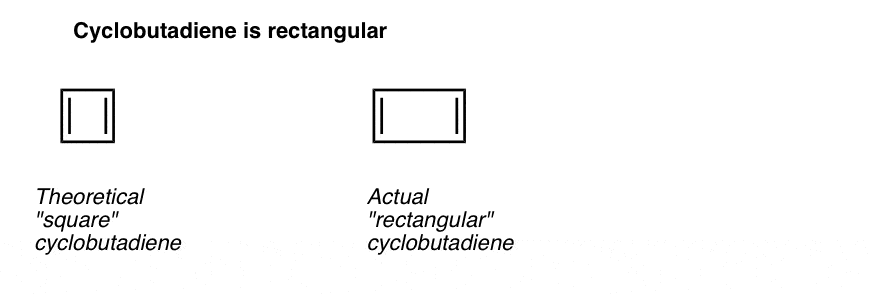
What this tells us is that even when a molecule fulfills all the conditions (cyclic, conjugated, flat, 4 pi electrons), the symmetric geometry is particularly unstable. [Note 5]. Very interesting!
5. An 8 Pi-Electron Example of an Antiaromatic Compound: Pentalene
So far all the examples we’ve seen so far had 4 pi electrons. You might be wondering: are there any examples of anti-aromatic molecules that have more than 4 pi electrons? Why, yes.
The molecule below is called “Pentalene”. It has been synthesized, but is only stable below –100 °C. Above this temperature it combines with another molecule of itself (reference). Another example of spectacular instability.

Pentalene has 8 pi electrons. This might set off some alarm bells of recognition. Remember how the number of pi-electrons in aromatic molecules followed the sequence (2, 6, 10, 14….) ?
This example suggests that the number of pi electrons in anti-aromatic molecules follows the sequence (4, 8, 12…)
6. What Makes A Molecule Anti-Aromatic?
So what do all of these molecules in this rogues’ gallery have in common?
Each of them is cyclic, conjugated, and flat – and when you count the number of pi electrons, it’s multiples of 4. So while aromatic molecules have (4n+2) pi electrons, the “rule” for anti aromatic molecules is (4n). (another way to look at it: the number of pi electrons will be twice an even number).
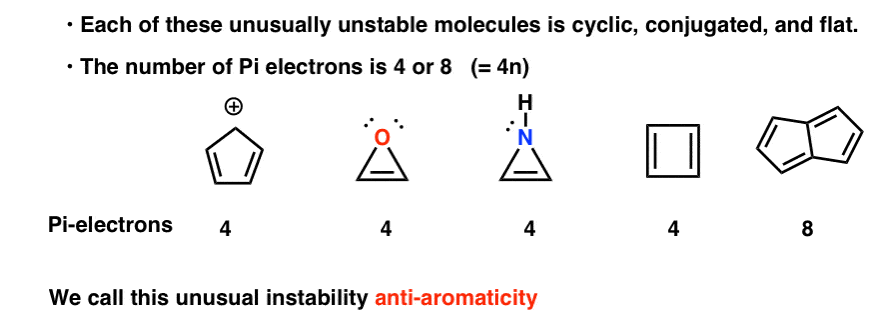
This unusual instability is called “anti-aromaticity”.
This means that we can now draw up three categories for molecules according to the following criteria:
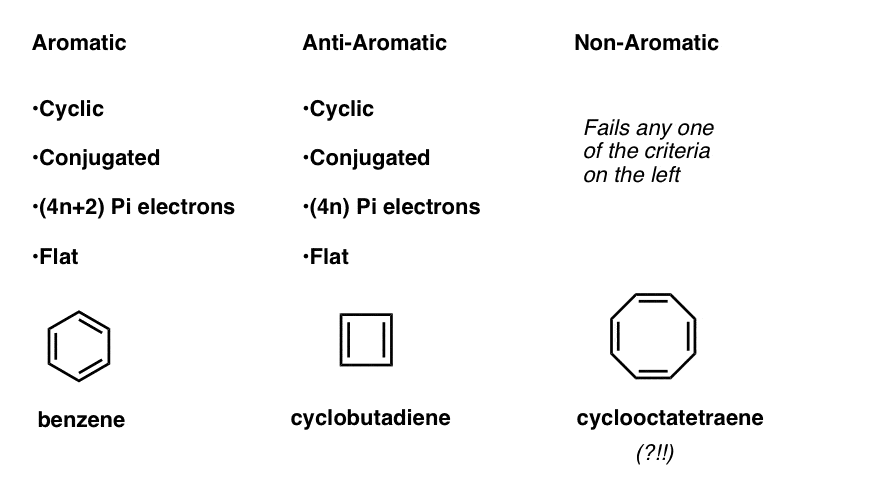
- Aromatic molecules are cyclic, conjugated, have (4n+2) pi electrons, and are flat.
- Anti-aromatic molecules are cyclic, conjugated, have (4n) pi electrons, and are flat.
- Non-aromatic molecules are every other molecule that fails one of these conditions.
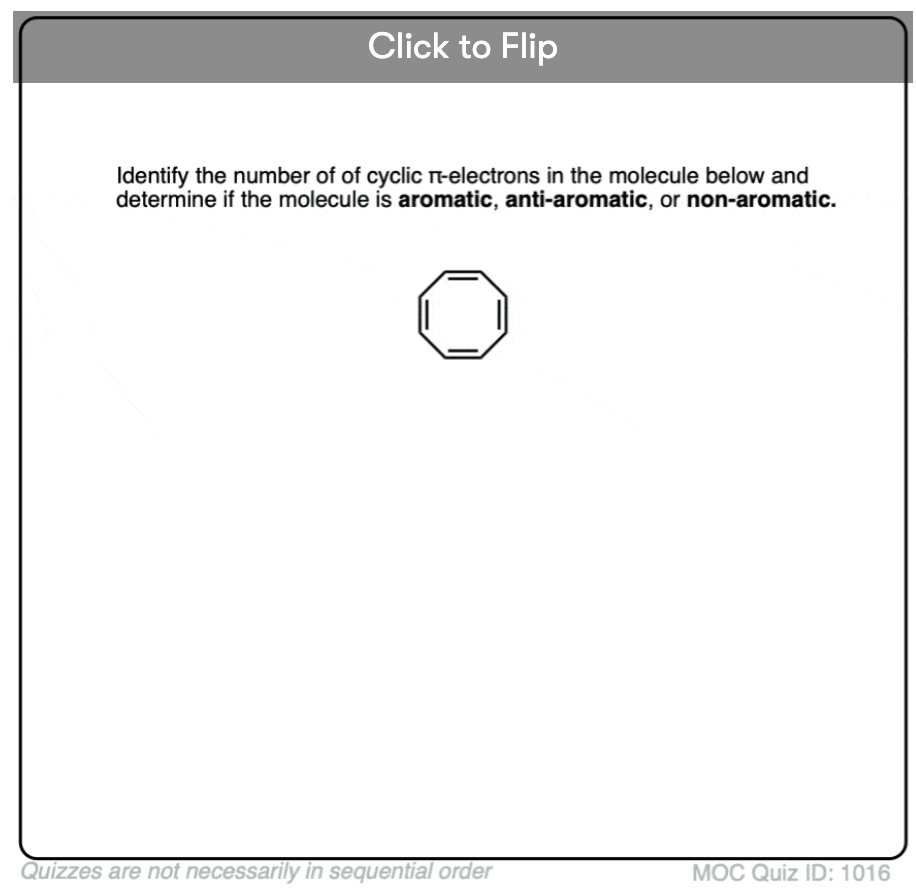
Wait a second – you might wonder why cyclooctatetraene is classified as “non-aromatic”. With 8 pi electrons (twice an even number) shouldn’t it be “anti-aromatic”?
7. Cyclooctatetraene “Escapes” Anti-Aromaticity Through Twisting Out Of Flatness
Think of fulfilling the conditions for “anti-aromaticity” as a bit like qualifying for an extremely punishing income tax. Given the opportunity to find a loophole to get out of the tax, would you do it? Probably.
Cyclooctatetraene is anti-aromatic only if it is flat. However, the relatively “floppy” structure of cyclooctatetraene allows for some flexibility. The bonds can rotate away from flatness such that the molecule adopts a “tub-like” shape, thereby avoiding the “antiaromaticity tax” of 18 12 kcal/mol that would be paid if all the p-orbitals on the molecule were conjugated with each other.
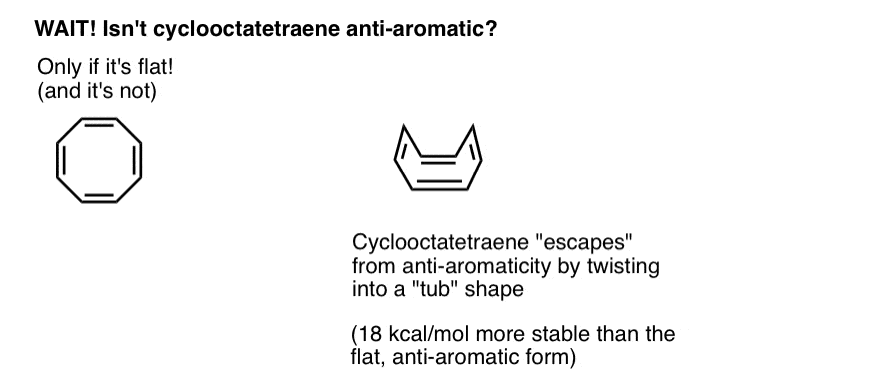
It turns out that cyclooctatetraene has been synthesized, is stable (you can buy it here, for example) and behaves like a “normal” alkene, undergoing addition reactions, hydrogenation, and so on.
Pentalene (above) which also has 8 pi-electrons, has a very rigid bicyclic structure that prevents bond-rotation away from flatness. Hence, it’s stuck in its anti-aromatic conformation.
Anti-aromaticity for molecules with more than 8 pi electrons is known, but very unusual. (Here’s a fun example of another “escape” from aromaticity for a molecule with 18 pi electrons.)
8. Conclusion: Antiaromatic Compounds
So far our treatment of aromaticity and anti-aromaticity has been purely descriptive and empirical. We’ve shown lots of examples, and given lots of rules, but missing from the discussion has been any deep explanation of “why“.
What’s so special about the benzene ring that makes it so stable? Why is it stable?
What’s so special about the cyclobutadiene system that makes it so unstable. Why is it unstable?
In order to answer these deeper questions, we’re going to have to step back and examine the molecular orbitals of these two molecules, and then come to a deeper understanding of aromaticity and anti-aromaticity.
That’s what we’ll do in our next post.
Thanks to Matthew Knowe for help in preparing this post.
Notes
Note 1. For simplicity, the image shown in the main article fudges on the actual reaction conditions. The actual experimental conditions are shown below. The solvent is propionic acid, and the Lewis acid silver perchlorate is added to assist with pulling off the iodine. [The idea is that Ag+ coordinates to the iodine, and then the C-I bond breaks. Silver iodide is extremely insoluble, and precipitates out of solution, driving the reaction towards completion].
Even under these conditions the cyclopentadienyl carbocation is not formed, which is a testament to its extreme instability.
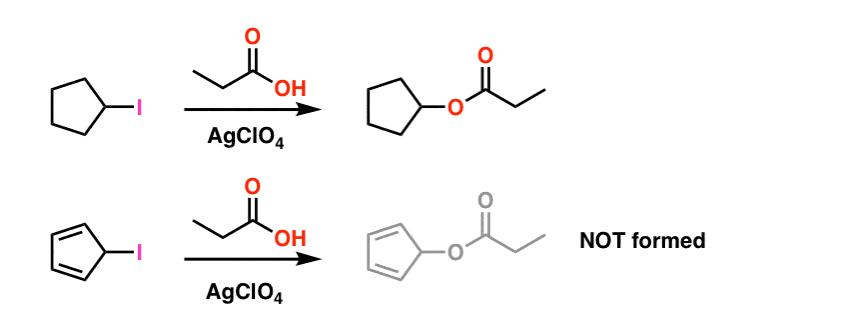
Note 2. Epoxidation on ethene (aka ethylene) is done to the tune of 15 million tons, annually. Epoxidation of acetylene to form oxirene is unknown.
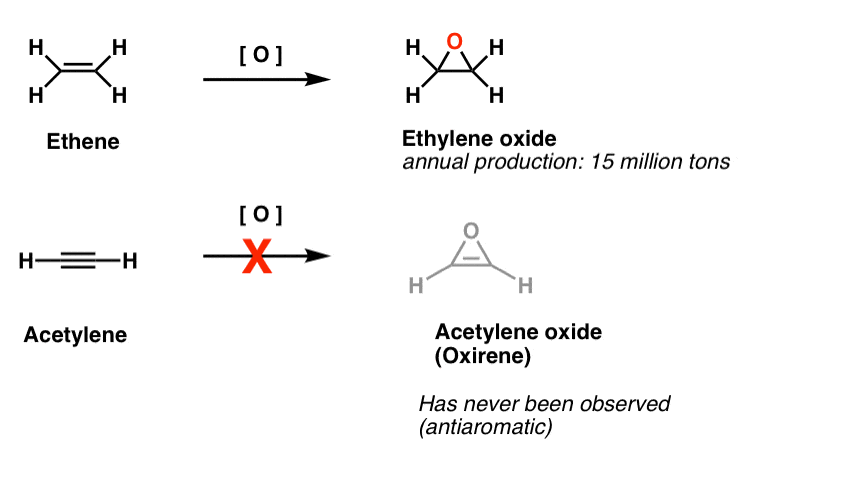
Fleeting evidence of the existence of oxirenes has been found. There is good evidence from isotope labelling studies that oxirenes are fleeting intermediates in certain Wolff rearrangements. There are also two examples of oxirenes that have been trapped at extremely low temperature, but decomposed above 35 Kelvin. [For the curious, here’s a review]
The fleeting existence of a 1H-azirine has been postulated in the addition of a nitrene to an acetylene, which quickly rearranges to a (stable) 2H-azirene (below).
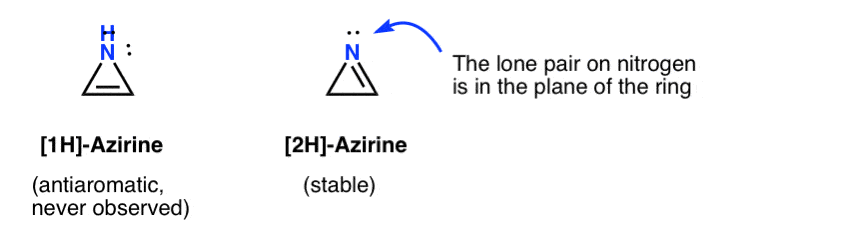
Note that in 2H-azirene the lone pair on the nitrogen is at right angles to the pi system, so this system is non-aromatic as opposed to anti-aromatic.
For more discussion, see March’s Advanced Organic Chemistry. I’ve been working off the 5th edition, pages 62-63.
Note 3. A very clever experiment determined that 1,2-dideutero cyclobutadiene has two isomers, not one (see below). This indicates that the double bonds in cyclobutadiene are not delocalized as they are in benzene, but are more like double bonds in a conventional diene.
Cyclobutadiene reacts with itself at 35 K to form a “dimer”, in an example of a Diels-Alder reaction.
Note 4. Yes, it’s true that the lone pair in azirene can be put into an sp3 hybrid orbital (below). This likely reduces the anti aromatic “penalty” somewhat, but the molecule is still incredibly unstable. Anti-aromaticity is the simplest way to account for this.

Note 5. The rectangular geometry is due to an effect known as the Jahn-Teller effect. Remember the Pauli exclusion principle, where electrons can’t have the same quantum number? Well, as we’ll see when we look at the molecular orbital diagram of cyclobutadiene, “square” cyclobutadiene has two “degenerate” electrons, i.e. they have the same energies and quantum numbers. This leads to a phenomenon known as “Pauli repulsion”, where elongation of the bonds occur until the energy levels of the two electrons are differentiated. Definitely an advanced topic.
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.

Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.

Become a MOC member to see the clickable quiz with answers on the back.
 Click to Flip
Click to Flip
(Advanced) References and Further Reading
- Transition-State Spectroscopy of Cyclooctatetraene
Paul G. Wenthold, David A. Hrovat, Weston T. Borden, W. C. Lineberger.
Science, 1996, 272, 1456-1459.
DOI: 10.1126/science.272.5267.1456
This study is the basis for the statement that the non-aromatic ground state of cyclooctatetraene is 12 kcal/mol below the “antiaromatic” conformation. - The Taming of Cyclobutadiene
Donald J. Cram, Martin E. Tanner, Robert Thomas
Chem. Int. Ed. 1991, 30 (8), 1024-1027
DOI: 10.1002/anie.199110241
This paper by Nobel Laureate Prof. Donald J. Cram (UCLA) describes the first spectroscopic characterization of cyclobutadiene. This is accomplished by enclosing the precursor in a hemicarcerand (cage complex), and then irradiating it with UV light, thus creating cyclobutadiene, which is unable to do anything since it is trapped. - Cyclobutadiene
Thomas Bally, Satoru Masamune
Tetrahedron 1980, 36 (3), 343-370
DOI: 1016/0040-4020(80)87003-7
This paper from 1980 reviews work done on cyclobutadiene up to that time. This is divided into 2 parts – experimental synthetic efforts and theoretical calculations. - Tetrahedrane and Cyclobutadiene
Günther Maier
Chem. Int. Ed. 1988, 27 (3), 309-332
DOI: 10.1002/anie.198803093
This paper reviews synthetic efforts towards tetrahedrane and cyclobutadiene – both molecules have a formula of C4H4. Only substituted (tetra t-butyl and tetra trimethylsilyl) tetrahedranes have been successfully synthesized and isolated to date. - Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes
F. Emerson, L. Watts, and R. Pettit
Journal of the American Chemical Society 1965, 87 (1), 131-133
DOI: 10.1021/ja01079a032 - Cyclobutadieneiron Tricarbonyl. A New Aromatic System
D. Fitzpatrick, L. Watts, G. F. Emerson, and R. Pettit
Journal of the American Chemical Society 1965, 87 (14), 3254-3255
DOI: 10.1021/ja01092a050
A bonus topic: cyclobutadiene can be stabilized with metals – the organometallic complex cyclobutadiene iron tricarbonyl is a stable, crystalline solid. As the authors mention, it is unlikely that free cyclobutadiene is generated in the synthesis. - Olanzapine.
The antipsychotic olanzapine adopts a “butterfly” shape to avoid antiaromatic effects, similar to cyclooctatetraene. This is apparently critical to its function. - Antiaromaticity
Ronald Breslow
Accounts of Chemical Research 1973, 6 (12), 393-398
DOI: 1021/ar50072a001
Interestingly, Prof. Breslow (Columbia) states in this account that he was the person to first suggest the term “antiaromatic” for especially destabilized cyclic compounds with 4n p electrons. He also cites other work by Prof. M. J. S. Dewar and others where the antiaromatic destabilization of cyclobutadiene is calculated to be between 18-33 kcal/mol relative to linear butadiene. - Experimental Determination of the Antiaromaticity of Cyclobutadiene
Ashok A. Deniz, Kevin S. Peters, Gary J. Snyder
Science 1999, 286 (5442), 1119-1122
DOI: 1126/science.286.5442.1119
This is a very rigorous paper that uses novel spectroscopic techniques to determine antiaromatic destabilization of cyclobutadiene. Relative to a hypothetical strain-less, conjugated diene reference, cyclobutadiene is destabilized by a total of 87 kcal/mol, 32 kcal/mol of which can be attributed to ring strain and 55 kcal/mol to antiaromaticity (compared with 21 kcal/mol for the aromatic stabilization of benzene). - Unsubstituted cyclopentadienyl cation, a ground-state triplet
Saunders, R. Berger, A. Jaffe, J. M. McBride, J. O’Neill, R. Breslow, J. M. Hoffmann, C. Perchonock, E. Wasserman, R. S. Hutton, V. J. Kuck
Journal of the American Chemical Society 1973, 95 (9), 3017-3018
DOI: 10.1021/ja00790a049
The cyclopentadienyl cation can only be prepared with difficulty, as this paper describes. Once it is formed, it has a triplet electronic ground state (with 2 unpaired electrons), and can be characterized by EPR (electron paramagnetic resonance) spectroscopy. - Oxirenes
Errol G. Lewars
Chemical Reviews 1983, 83 (5), 519-534
DOI: 10.1021/cr00057a002
How do we that if a compund is flat or not?
It’s generally a good assumption that if a molecule is cyclic, has 6 pi electrons, and is conjugated all around the ring, that it will arrange itself to be flat.
Some antiaromatic compounds with 4- and 5- membered rings can’t really distort themselves out of flatness (too much ring strain!) , but larger ones (like cyclooctatetraene) can, and for that reason, cyclooctatetraene is considered to be “non aromatic” instead of antiaromatic.
sorry, of course the azirinium cation!
That would be an interesting species to try to make. 6-electron nitrogen species are extremely unstable, but that one might have a shot. Do you know if anyone has tried making it?
Interestingly, the 2H-Azirine is actually an aromatic compound! (flat, 2 pi electrons, cyclic, conjugated)
One of the rings is aromatic only, the one with the three double bonds, the other ring as it is not conjugated or flat is simply non aromatic. Even if only one ring in a poly cyclic compound is aromatic the entire thing is considered aromatic.
In my aromatic compounds test , In a question numbers of aromatic compounds are asked . In that dialin was considered as aromatic compound (in jee adv 2017 ) . Why they considered it as aromatic if this has not all atoms conjugated and not obey huckels rule also ?
thank you for all this info!! I have a question about cyclobutadiene though, can we say it’s anti-huckel? and the formula 4n, does n represent the number of double bonds? or the number of pi bonds only? just a little confused on that part
Hey, where’d you come up with the value of 18 kcal/mol for the energy difference between planar and tub shaped COT? If I remember right, the bond-alternated structure of planar COT is more like 12 kcal/mol above the tub. The octagonal structure is about 2 kcal/mol higher than that.
These values can be deduced based on the old Paquette kinetics measurements for inversion and bond-shifting, or more directly from the photoelectron spectroscopy.
On the cyclopentadiene, what does the + charge indicate? How does that compare to a cyclopentadiene with a negative charge?
The + charge indicates that one carbon is a carbocation. That is, a carbon having only 3 bonds, 6 valence electrons, and an empty lone pair.
Hi! How would be know that cyclooctatetrene is flexible and therefore nonplanar? Are there other molecules that are non aromatic because of their flexibility?
Well, one way would be to make a model. Models are fairly reliable for determining such things. If you make a model of cyclooctatetraene you will see that it’s pretty floppy and can form a tub shape quite easily.
As for other examples… well, here’s one: “The Great Escape” From Antiaromaticity… https://pubs.acs.org/doi/abs/10.1021/ja0291991
There’s a typo: “Cyclobutadiene reacts with itself at -35 K to form a “dimer”, in an example of a Diels-Alder reaction.” Maybe -35 degrees C, but not negative Kelvin.
Ouch. Thanks for correcting that!