Carbohydrates
Reducing Sugars
Last updated: March 21st, 2025 |
Reducing Sugars
- Reducing sugars are small carbohydrates (usually containing one or two sugar units) that are capable of acting as reducing agents towards metal salts such as Ag+ or Cu2+ .
- These metal salts have historically been used for testing purposes because they oxidize aldehydes and give a clear color change after being reduced.
- The reason sugars can react with these salts is that their cyclic hemiacetal functional group is in equilibrium with an acyclic aldehyde. Acetals do not react.
Therefore a quick way of telling if a sugar is a reducing sugar is to notice whether or not it has a hemiacetal.
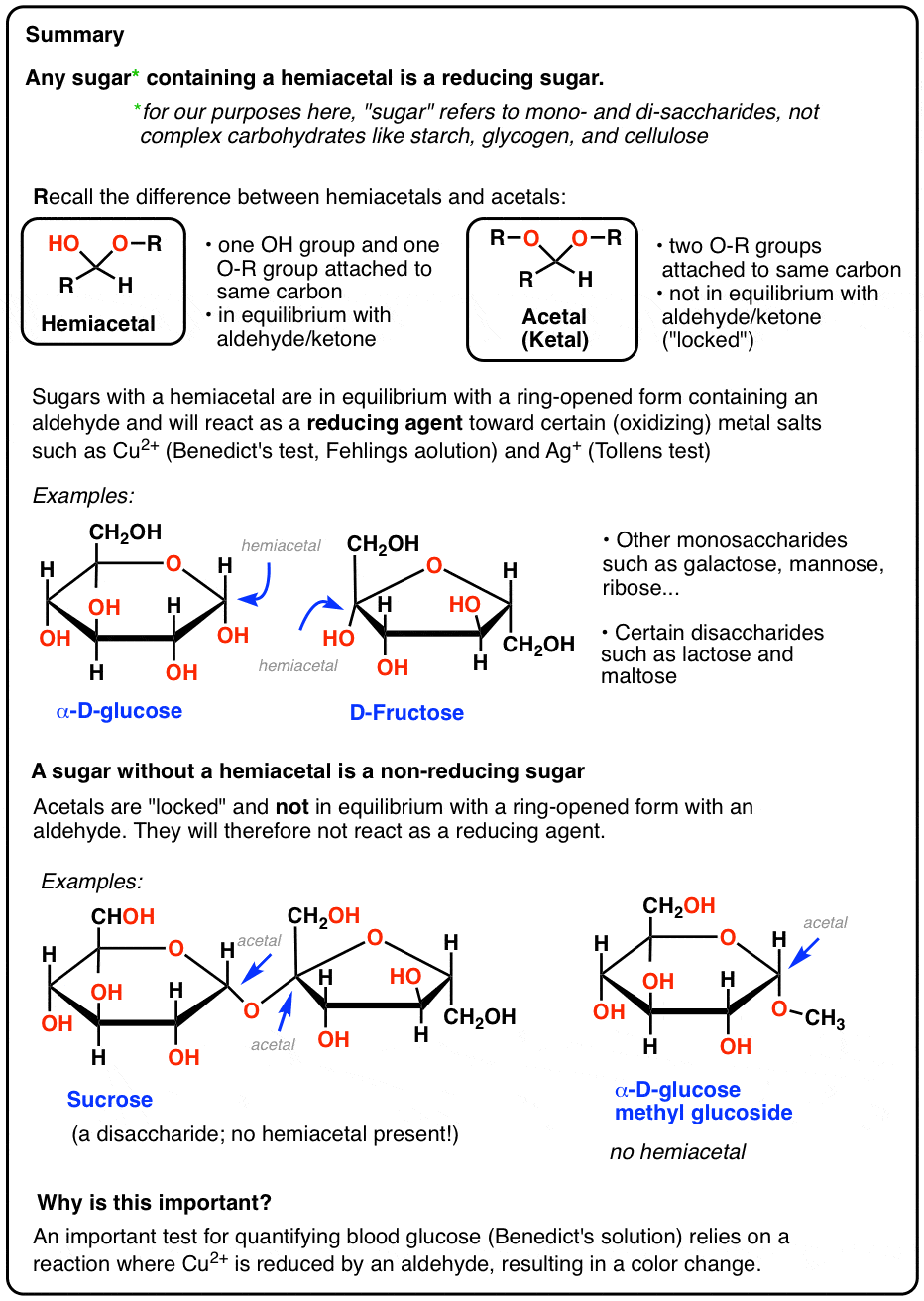
Table of Contents
- Before We Talk About Reducing Sugars, The Chemistry Of “Peeing On The Stick”
- Benedict’s, Fehlings and Tollens’ Tests: Three Visual “Tests” For The Presence of Aldehydes
- Reducing Sugars: Sugars With A Hemiacetal Functional Group Give Positive Tests Since They Are In Equilibrium With An Open-Chain Aldehyde
- So What Isn’t A Reducing Sugar?
- Saccharides Lacking A Hemiacetal Are NOT Reducing Sugars
- Complex Polysaccharides With A single Hemiacetal Unit (e.g. Starch) Are Not Reducing Sugars
- Test Yourself On Reducing Sugars
- The Chemistry Of The Benedict, Fehlings and Tollens Tests
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Before We Talk About Reducing Sugars: The Chemistry Of “Peeing On The Stick”
Q. Can you think of a situation where it might be useful to be able to measure the concentration of glucose in a solution (especially in blood or urine) ?
A. Diabetes. Once you have a way of quickly and easily measuring the concentration of sugar, then you can determine how much insulin is needed to counteract it.
Next question. What would be an easy, visual way to detect the presence of glucose? Especially something that doesn’t require you to be an expert chemist?
Ideally, you’d like a chemical reaction that results in a color change.
Think about pregnancy tests: you just pee on a stick and know within a few minutes whether you’re pregnant. You don’t need to know any chemistry. It’s brainless.
A test for blood sugar suitable for diabetics should have a similar ease of use.
This brings us (via aldehydes) to the topic of reducing sugars, since they are the basis of a historically important color-based test for blood glucose.
2. Three Visual “Tests” For The Presence of Aldehydes: Benedict’s, Fehlings, and Tollens’ Tests
Before we get to sugars, let’s talk about the oxidation of aldehydes.
We’ve seen previously that aldehydes are a functional group that can be oxidized relatively easily to carboxylic acids. For example, oxidation of alcohols with a “strong” oxidant like chromic acid (H2CrO4) results in an aldehyde that is quickly oxidized further to a carboxylic acid.
During this process, the aldehyde is oxidized and the oxidizing agent is reduced. Another way of framing this is to say that the aldehyde is the reducing agent in this process.
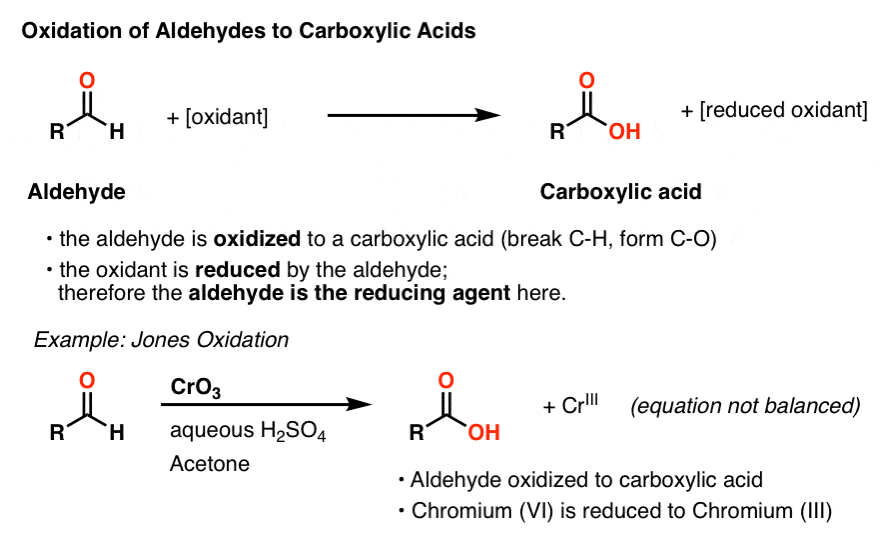
The list of reagents that can be used to oxidize aldehydes to carboxylic acids is loooong. Of these, a few methods stand out in providing a particularly clear visual indication that the reaction has proceeded to completion. [Note 1]
Three “visual” tests for aldehydes that you might encounter in an introductory organic chemistry lab are the following:
- Fehling’s solution, where an aldehyde changes the color of a blue Cu(II) solution to red Cu(I) [as Cu2O].
- Benedict’s solution a slightly modified version of Fehling’s solution
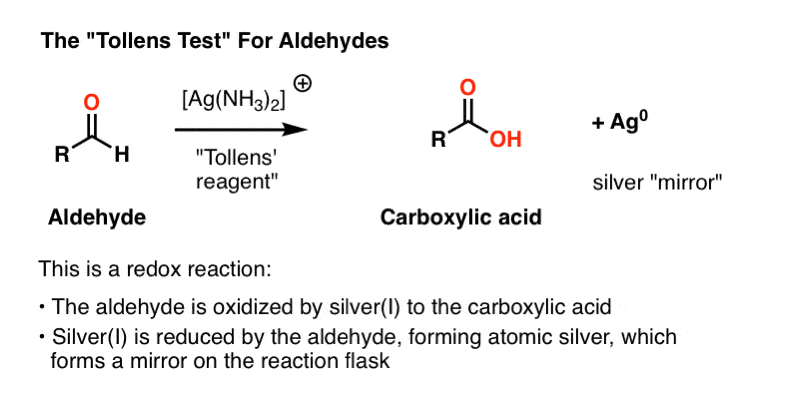
- Tollens’ test, where aldehyde oxidation results in a beautiful “mirror” of silver metal to precipitate on the reaction vessel.
[details on the actual chemistry of these tests at the bottom of the post [jump]

Importantly, ketones don’t react under any of these conditions. The above tests were also a useful way of distinguishing aldehydes from ketones in the dark days before IR and NMR spectroscopy made this routine.
So what does this have to do with sugars? Let’s go back to Yelling Mode:
3. Reducing Sugars: Sugars With A Hemiacetal Functional Group Give Positive Tests Since They Are In Equilibrium With An Open-Chain Aldehyde
As we’ve seen, glucose is in equilibrium with an open-chain (or “linear”) form containing an aldehyde.
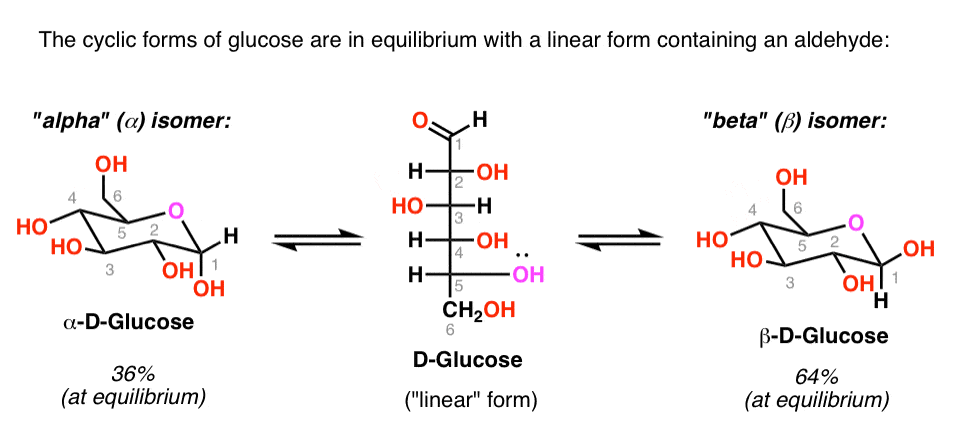
The concentration of aldehyde at any given time is small (<1%), but long-lived enough to be trapped with the right reagent.
This means that glucose will give a positive test with Benedicts’ reagent, Fehlings solution, or the Tollens test, and the aldehyde will be oxidized to a carboxylic acid.
Voila! A simple color change tells you if glucose is present!
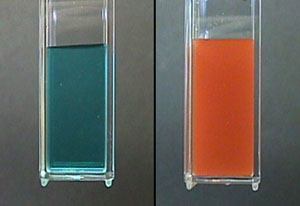
What about quantification?
It’s nice to have a quick visual test for glucose. But what if we want to determine the exact concentration of glucose in a solution of, say, urine or blood?
In this case, a slightly different formulation of Benedicts solution is used [Note 2] which results in a colourless precipitate rather than a red color. A solution of the sample to be analyzed is added, via buret, to a flask containing a known amount of Benedicts solution until the blue color of the Cu(II) disappears. The unknown sample is then calibrated using a 1% solution of glucose. [Full details here]
Benedicts assay was the method of choice for quantifying glucose for over 50 years. One researcher recalls that all inductees into the U.S. army during World War II had their urine tested for sugar with Benedict’s Solution.
In recent times, however, the use of Benedict’s solution has been supplanted by enzymatic methods such as glucose oxidase. Why?
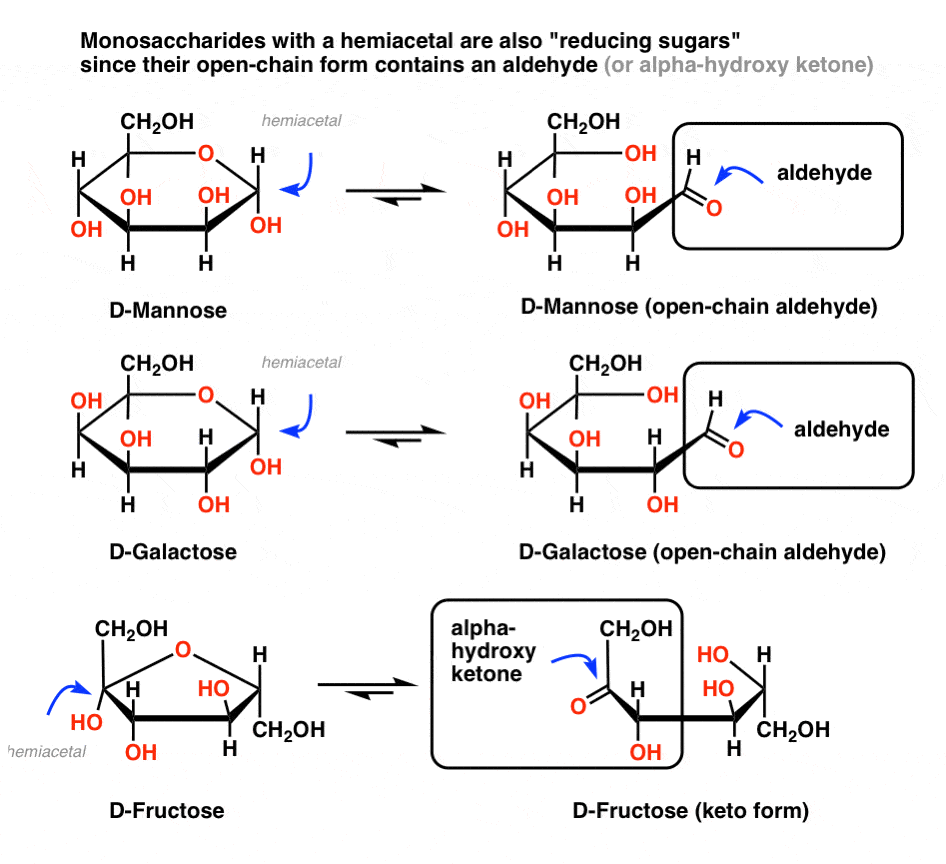
The Benedict test isn’t specific for glucose; it just tells you if an aldehyde is present. So it will also give a positive test for other reducing sugars.
In short, any sugar* (*mono- or disaccharide) with a hemiacetal will also give a positive test, since these sugars are in equilibrium with an open-chain aldehyde. So if the blood/urine contains common monosaccharides like mannose, galactose, or fructose, these will deliver a positive test. In other words, those sugars are also reducing sugars.
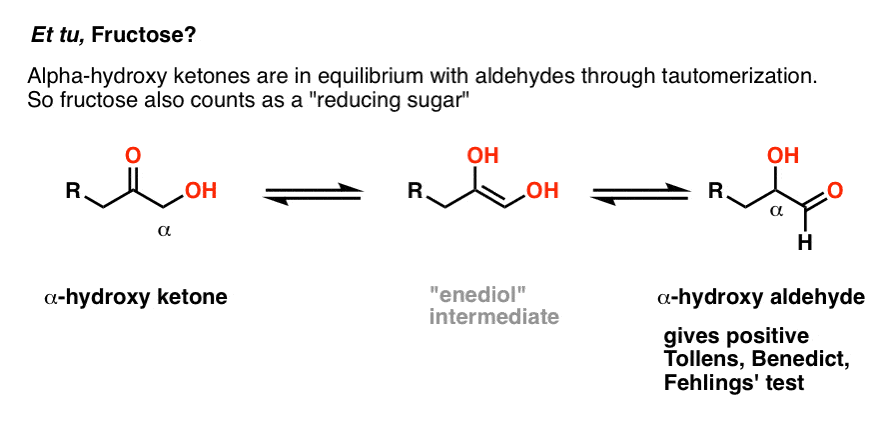
Hold on for a second. Et tu, fructose?
Ketones aren’t supposed to oxidize under these conditions! So why does fructose give a positive test?
Great question. Although fructose is a keto sugar, and ketones generally give a negative test with the Benedict, there is an exception. If the carbon adjacent to the ketone carbon (the “alpha carbon”) contains a hydroxyl group, the ketone will be in equilibrium with an aldehyde through tautomerization (just for the record, this is called an “enediol rearrangement”). [Note 3]
Likewise, some disaccharides such as maltose and lactose contain a hemiacetal. They are also reducing sugars that give a positive Fehlings, Benedict, or Tollens test (picture of lactose positive test is further below).
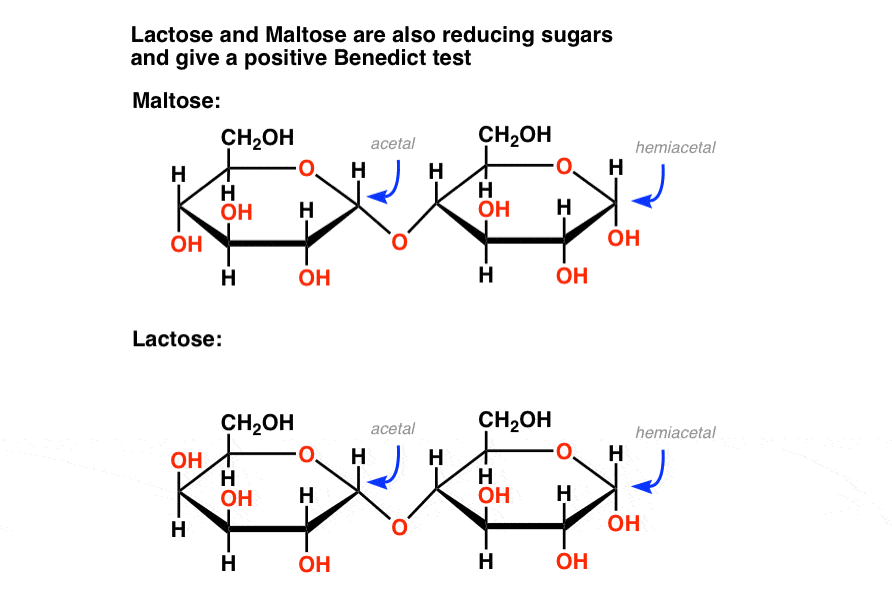
The bottom line is that Benedicts’ reagent quantifies reducing sugars which includes not just glucose but also mannose, lactose, maltose, fructose, and others. That means that the test isn’t as specific as we’d like!
4. So what isn’t a reducing sugar?
So far, it seems like every sugar we’ve encountered is a reducing sugar. So it’s fair to ask: when is a sugar not a reducing sugar?
Two main cases:
- mono and di-saccharides which lack a hemiacetal
- polysaccharides where the ratio of hemiacetals to acetal linkages is very low (e.g. starch)
5. Saccharides That Lack A Hemiacetal Are Not Reducing Sugars
We saw at the top of the post that hemiacetals are in equilibrium with an aldehyde or ketone. In contrast, acetals (ketals) are locked in place and can only be converted back to the aldehyde or ketone with aqueous acid. That’s why they make great protecting groups for aldehydes/ketones.
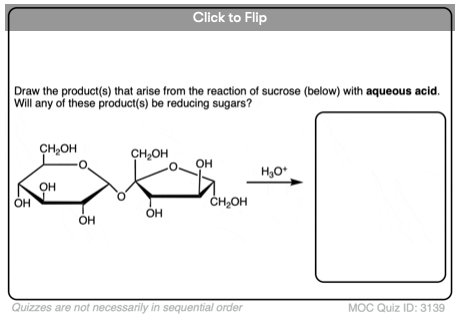
The poster child for a non-reducing sugar is sucrose, a.k.a. table sugar.
Sucrose is a disaccharide of glucose and fructose. See if you can find a hemiacetal in its structure, below:
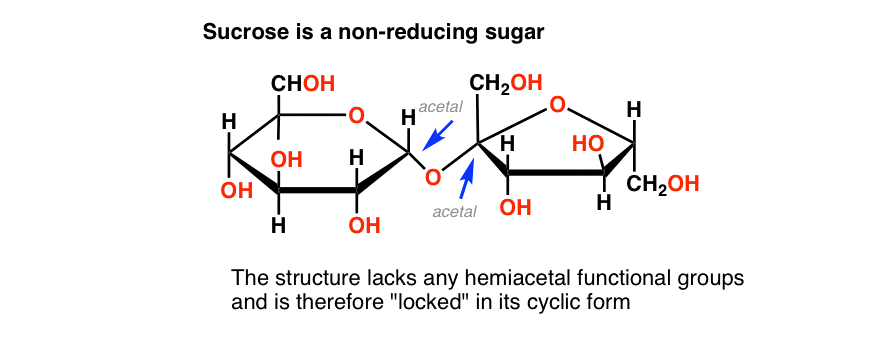
There isn’t one! Sucrose only has acetal groups, and since acetals don’t open up to aldehydes under the basic conditions present in the Benedict test, sucrose isn’t a reducing sugar. [Note 4]
Sucrose gives a negative test (blue) to the Benedict solution.
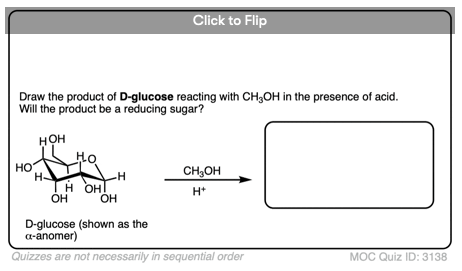
Another example of a non-reducing sugar are the so-called “glucosides” of common sugars, such as glucose methyl glucoside, below. This is obtained by heating glucose in acidic methanol.
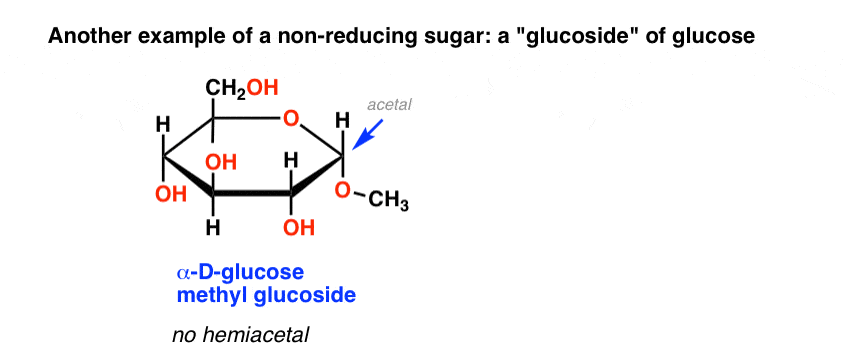
Lacking a hemiacetal which could open to an aldehyde, this methyl glucoside also gives a negative Benedict’s test.
6. Complex Polysaccharides Which Only Have A Single Hemiacetal Unit Don’t Count As Reducing Sugars (e.g. Starch)
Sugars are able to form long chains with each other in arrangements known as polysaccharides. Common examples of polysaccharides are starch, cellulose, and glycogen.
The vast majority of individual sugar units in these polysaccharides are joined to each other via acetal (“glycosidic“) linkages. Hemiacetals are present, but only at the termini of the polymer.
Starch, for example, generally has about 300-600 individual units of glucose, but only one unit (the terminus) has a hemiacetal.
One hemiacetal “needle” in a haystack of “acetals” is not enough to give a positive test for reducing sugars. Therefore these polysaccharides are not considered reducing sugars. For example, starch gives a negative test (see below).
Here is an example of Benedict’s test with lactose, starch, glucose, fructose, and sucrose (image source).

Note that starch and sucrose are blue, classifying them as non-reducing sugars.
That’s enough about what classifies a “reducing sugar” from a “non-reducing sugar”.
Here’s the last step. Test yourself. What’s a reducing sugar and what is not?
7. Test Yourself On Reducing Sugars
Make sense? Quiz yourself on whether the following sugars are reducing sugars or non-reducing sugars.
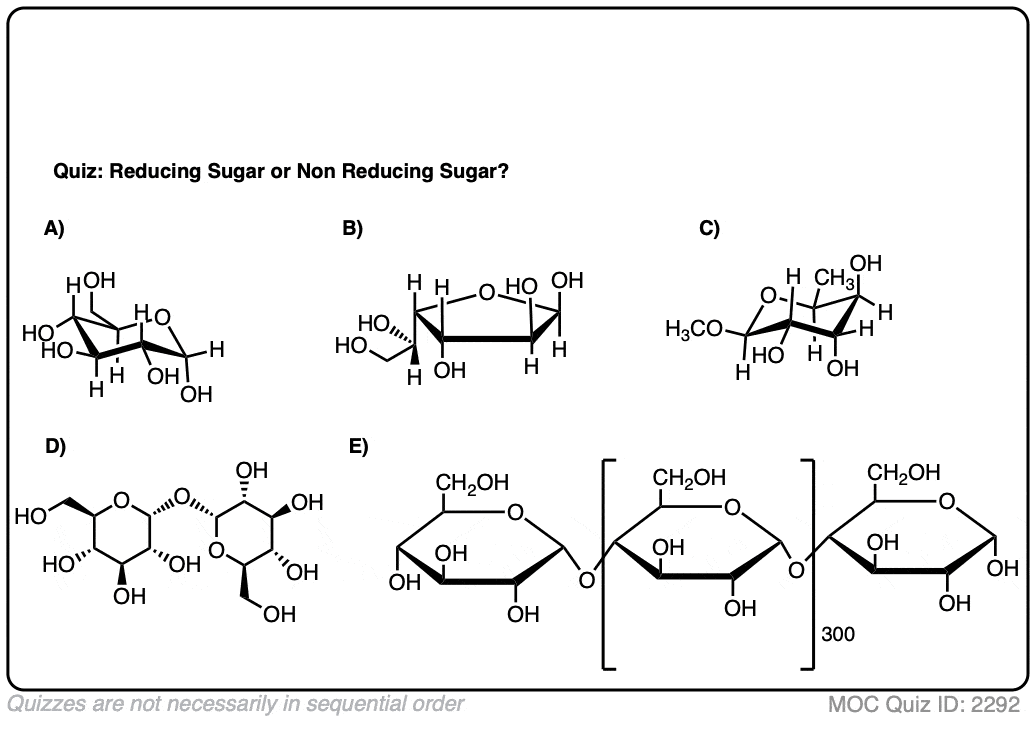 Click to Flip
Click to Flip
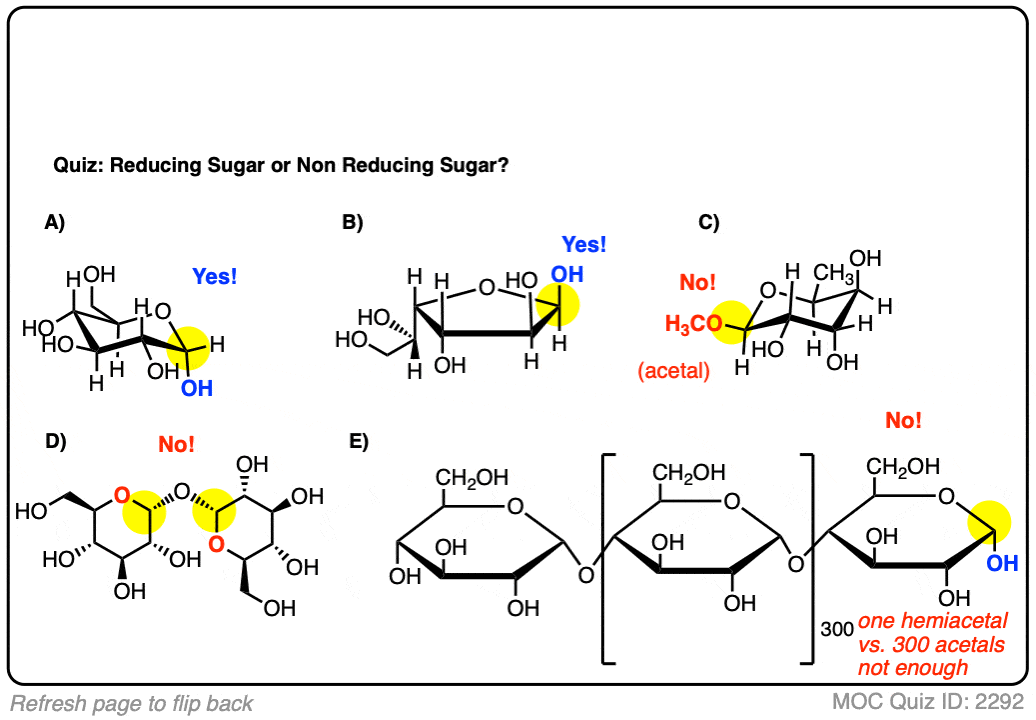
If you don’t need to know anything else other than “what’s a reducing sugar”, you’re done here.
But if you want to go further down the rabbit hole, I invite you to read further to learn about…
8. The Chemistry of the Benedict, Fehlings, and Tollens tests
So what’s actually going on in the Benedict, Fehlings and Tollens tests? Let’s discuss the details of the chemistry.
One thing about all three tests is that the active reagent is not particularly bench stable and has to be freshly prepared.
Fehling’s Solution
For Fehling’s solution, one starts with bright blue copper(II) sulfate, sodium hydroxide, and potassium sodium tartrate (otherwise known as Rochelle’s salt). The purpose behind using the tartrate is that it coordinates to the copper(II) and helps prevent it from crashing out of solution.
Once prepared, the substance to be analyzed is added, and the mixture is heated for a brief period.
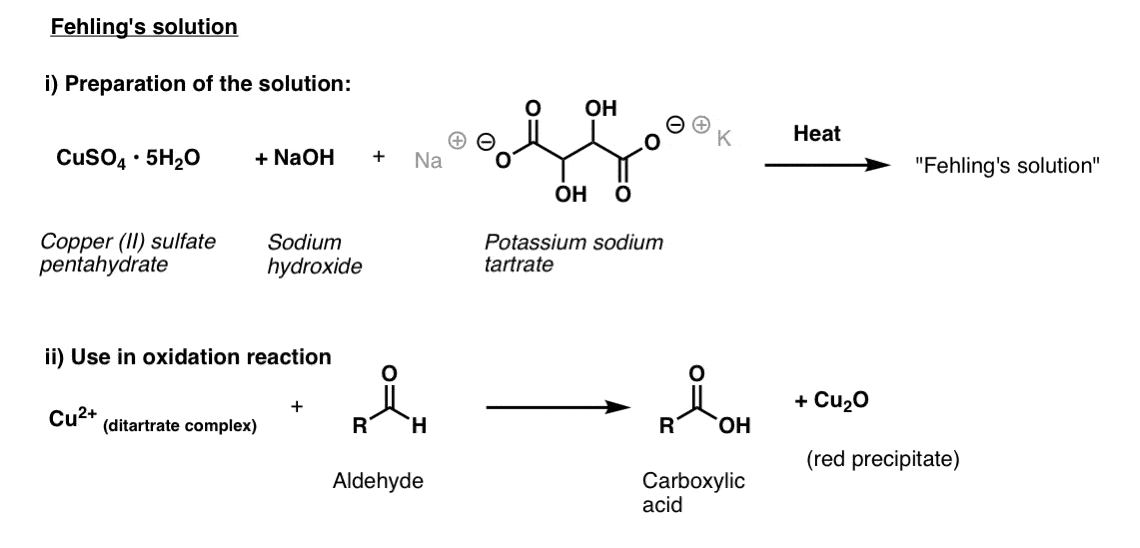
This results in a carboxylic acid and red Cu(I) which precipitates out as copper(I) oxide.
The structure of the active species in Fehling’s solution has been determined; it’s a square-planar copper complex attached to two tartrate ligands. [link – paywall]
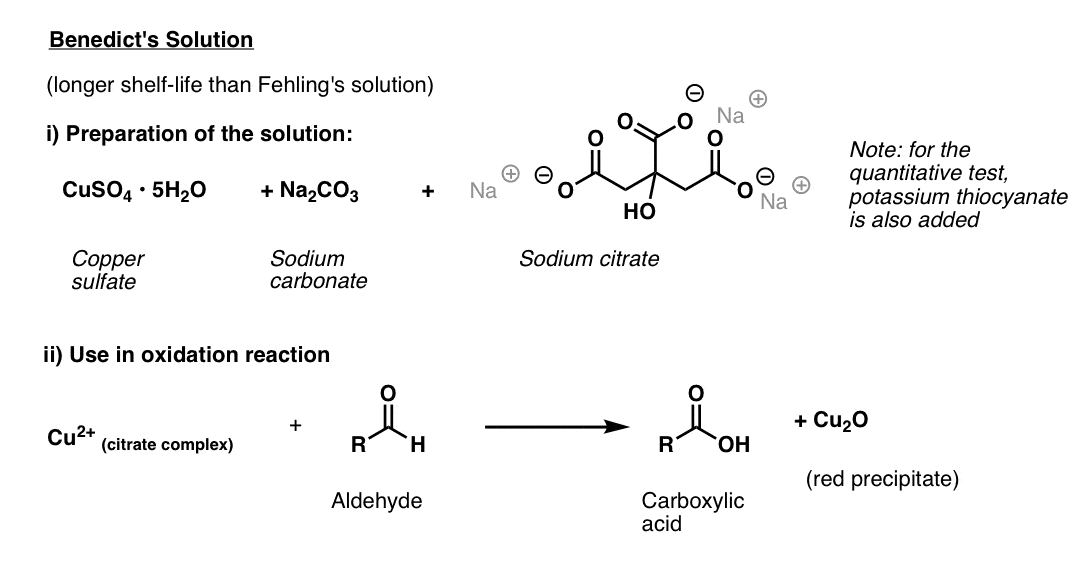
Benedict’s Solution
Benedict’s solution is a slight variation of Fehling’s solution that uses citrate instead of tartrate, which provides better stability for the copper(II).
Like Fehling’s solution, it is best made fresh. The ingredients are copper(II) sulphate, sodium carbonate (note: hydroxide is also needed! – see reference), and sodium citrate. (Note: in the quantitative test, potassium thiocyanate is added, which results in a colourless white precipitate).
The test is performed by adding the substance to be analyzed and heating briefly.
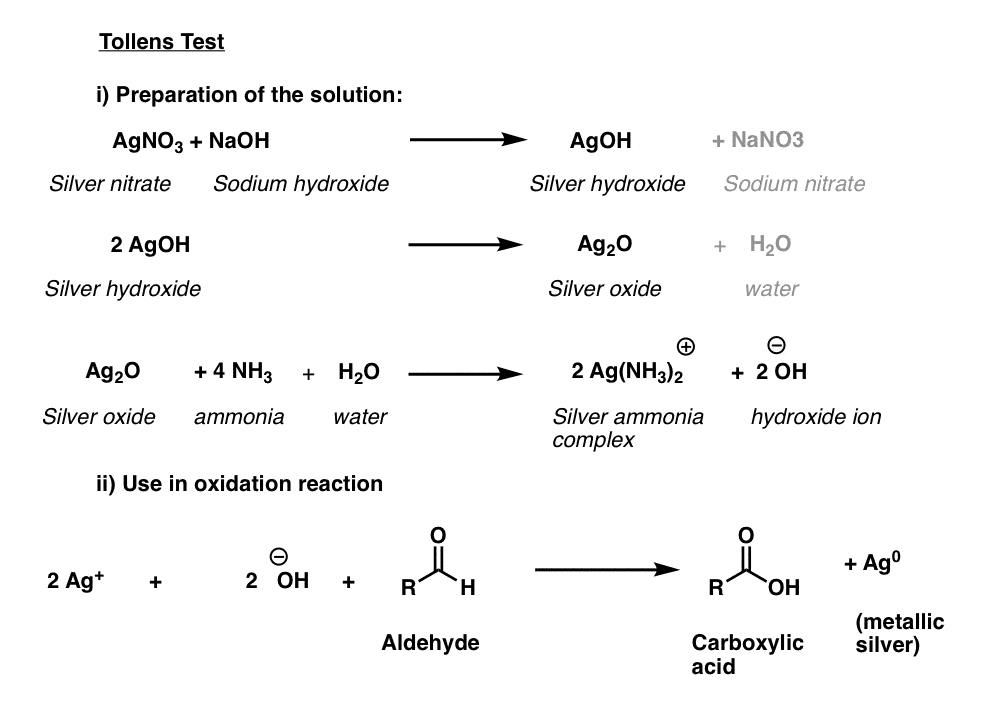
Tollens’ Solution
The active ingredient in the Tollens test, [Ag(NH3)2]+ , does not have a long shelf life and like the Fehlings’ and Benedict solutions is best prepared fresh.
The first three lines below describe the procedure. Silver nitrate is converted to silver hydroxide, which forms silver (I) oxide, Ag2O. Then, addition of aqueous ammonia (NH3) results in formation of the silver-ammonia complex which is the active oxidant.
The sample to be tested is then added to the freshly prepared active oxidant in a basic solution. A positive test results in a beautiful mirror of silver metal being precipitated out on the reaction vessel. (A variant of this procedure is used for the preparation of mirrors).
How Does It Work?
The first thing to note is that all of these procedures occur in basic solution.
Why? There’s at least two good reasons for this that we can talk about.
- First, acidic conditions might hydrolyze any acetals present to hemiacetals, giving a false positive test.
- Secondly, base considerably speeds up the rate of ring-chain tautomerism (i.e. interconversion between the cyclic hemiacetal form and the linear aldehyde form).
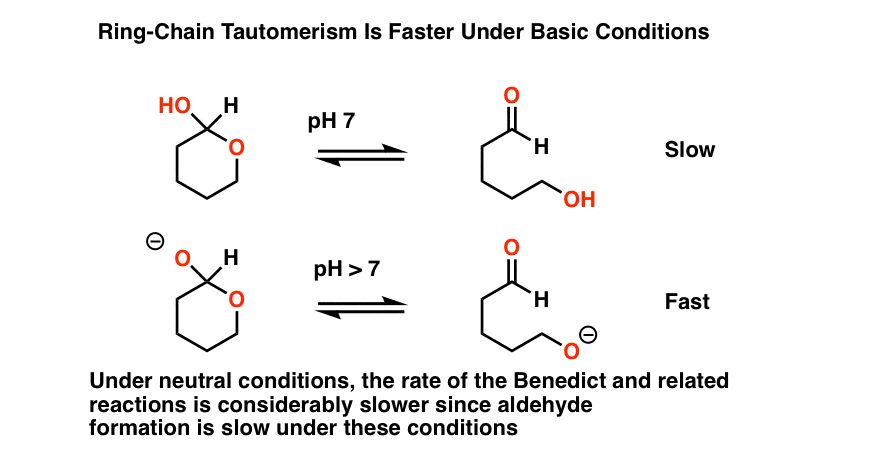
Bottom line here is that adding base has the effect of increasing the concentration of the starting aldehyde.
The Mechanistic Details Are Murky And You Will Not Find Them In Any Introductory Textbook
I can’t find a single instance of the mechanism for the Fehlings or Benedict solutions being elucidated conclusively online. If I am wrong, please tell me (leave a comment).
There is a third reason for the use of base, although I’m not very keen on talking about it. You might notice we haven’t mentioned the mechanisms of any of these reactions. That’s because the exact mechanisms have been tricky to elucidate. One of the key steps involved in the mechanism of each reaction seems to be a process called, “single-electron transfer” which is essentially when the metal salt slurps a single electron off of the substrate, creating a free-radical and/or carbocation.
One of the access points for the initiation of a single-electron transfer reaction is a carbon-metal bond, which can be achieved through base-promoted formation of an enolate.
That requires that the aldehyde have a proton on the alpha carbon (i.e. be “enolizable”). It turns out that Fehling’s solution does a crap job with testing for benzaldehyde, which lacks any protons on the alpha-carbon and can’t be enolized. Thus it would appear that the reaction needs to proceed through an enol.
However, Fehling’s solution also oxidizes formaldehyde to formic acid and thereon to carbon dioxide, and this process can’t possibly proceed through an enol/enolate intermediate.
So it is likely that a variety of mechanistic pathways can be in operation.
What might a mechanism look like?
Maybe, possibly, something like this?
To see a popup image, hover here or or click on this link to the image.
[The key step here would be generation of a carbocation on the alpha-position of the aldehyde, which accepts a hydride from a deprotonated hydrate-like intermediate, leading to formation of a carboxylic acid.]
If anyone else has a better idea, please feel free to comment below.
Notes
Image sources: Benedicts solution. Fehling’s solution. Tollens test.
Note 1. This isn’t to say that they’re the most practical methods for preparing carboxylic acids from aldehydes. When chemists want to prepare a carboxylic acid from an aldehyde in good yield, they don’t use any of these three processes. The standard way to do it is the Pinnick oxidation.
Note 2. The quantitative test apparently employs potassium isocyanate, which results in a colourless precipitate.
Note 3: It’s likely that the enediol intermediate is actually the species that reacts with Cu2+ in the initial step of the mechanism that leads to the aldehyde. See the mechanism section.
Note 4. One thing to note: if sucrose is heated with aqueous acid before a Fehlings/Benedict/Tollens test, a positive test will result. That’s because the acetal linkages will be hydrolyzed by aqueous acid to produce the two constituent sugars of sucrose (glucose and fructose) which are themselves reducing sugars.
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
- The Species of Fehling’s Solution
Thomas G. Hörner, Peter Klüfers
J. Inorg. Chem. 2016, 12, 1798-1807
DOI: 10.1002/ejic.201600168
Even though the reaction equation of Fehling’s test may look simple on paper, the species involved are actually quite complex! - The subjection of glutaraldehyde to the Tollens test
William D. Hill
Journal of Chemical Education 1990, 67 (4), 329
DOI: 1021/ed067p329
Dialdehydes will also give a positive Tollens test (silver mirror precipitate). - Tollens’s test, fulminating silver, and silver fulminate
Ian D. Jenkins
Journal of Chemical Education 1987, 64 (2), 164
DOI: 10.1021/ed064p164
The Tollens test is commonly carried out in undergraduate organic chemistry laboratories, with carefully tested procedures. The procedures have to be robust because the Tollens reagent can be explosive, as this note explains. - The Fehling and Benedict tests
Ralph Daniels, Clyde C. Rush, and Ludwig Bauer
Journal of Chemical Education 1960, 37 (4), 205
DOI: 10.1021/ed037p205
This note is interesting because the authors show that the Fehling and Benedict tests are specific for the hemiacetals in reducing sugars – they fail when used with simple aliphatic aldehydes. - A correction on the Benedict test
William D. Hill
Journal of Chemical Education 1982, 59 (4), 334
DOI: 10.1021/ed059p334
Several textbooks use Na2CO3 as a base in the Benedict test, but according to this note, NaOH is required for formation of Cu2+
Hi
In section six of Reducing Sugars there appears to be an image missing after this sentence:
Here is an example of Benedict’s test with lactose, starch, glucose, fructose, and sucrose (
Yes, good catch! Thank you, fixed
Good morning!
Thank you again for these courses and bravo for the quality of your pedagogy!
NB: I point out a schema error in §6
I love this nice explanation.
hi sir can i ask if im correct c is reducing sugar and e is also reducing sugar?
I have a suggestion for a minor correction.
The C-2 carbon of Fructose in a cyclic form is not hemiacetal but a “hemiketal”.
Likewise the same carbon in sucrose is “Ketal” and not Acetal. However, the notation for the Glucose part is correct both in monosaccharide as well as disaccharide.
Hi Jame
thanks for your good explanation. Can you give answers for quiz that is at part 7?
Wow, thank you for this great resource!!!
This website is phenomenal. Mr. Ashenhurst teaches these topics so much better than they do at school!
Keep up the great work!
Thanks Soumak, I’m glad you find the website useful. James
Hello James,
And thanks again for your very clear explanations. I have a question regarding reducing sugars as excipients with drugs. Some carbohydrates (reducing sugars) form covalent bonds with drugs that contain primary and secondary amines. So I have been looking at the Maillard reaction which breaks down the hydroxyl group of a reducing sugar and then forms Amadori products. I have been searching for a reaction mechanism for the Amadori adduct formed from dexamphetamine and lactose.
I was wondering if you could perhaps give a generic mechanism or a similar example. I note you give two examples of lactose and maltose as disaccharides that are reducing sugars as they contain the hemiacetal. I was wondering if you could give a general mechanism reacting with a primary amine such as R-NH2?.
Thanks again,
Peter
Hi Peter- I think what you’re looking for is 1) opening of the ring to give the linear product , and then 2) formation of an imine from the aldehyde. That’s the first step in the Maillard. The whole mechanism of the Maillard is here on wikipedia, https://en.wikipedia.org/wiki/Maillard_reaction , whereas I cover formation of imines here: https://www.masterorganicchemistry.com/2010/05/24/imines-and-enamines/
Great posts. Thoroughly enjoy. Wish you had been teaching when I took organic chem.
B.S. Chem, UNC-Chapel Hill, NC, 1966
Thank you Jerry. Much obliged. Glad to have you as a reader.
Also, your hypothetical mechanism looks black, FYI :-)
This is going to be during the pending Marie Kondo-ing of the site.
Thank you very much for your posts they are very helpful. Above in the first “box” you have written “Fehlings aolution).” I thought you might want to change that to solution.
Blessings and thanks again!
What if a product contains high percentage of reducing sugar? What does it denotes?I have prepared a product which contains 22. 16% reducing sugar and 3.26% non reducing sugar
I’m not a doctor but what you’re likely looking for is the presence of blood glucose vs. glycosides.
Brilliant James. A truly useful and imaginative resource.
Glad you’ve found it helpful Ted.
Great work, much appreciated!
Happy to help, Safari. Nice name BTW.
I wish the chemistry back in school was explained this way;) Cheers!
Thanks!
A very enjoyable post! Some minor problems, though:
– “Saccharides That Lack Without A Hemiacetal Are Not Reducing Sugars” – something’s gotta go!
– “The vast majority of individual sugar units in these polysaccharides are joined to each other via acetal (“glycosidic“) linkages Hemiacetals are present, but only at the termini of the polymer.” – a dot (a.k.a. full stop, period, baseline dot) is missing.
– The image showing the chemistry for the Benedict’s test is actually a copy of the one above, for the Fehling’s test.
– In the 3rd reaction of the Tollens test, the hydroxide ion is shown with the negative charge next to the hydrogen atom. In the 4th reaction, the charge is on the oxygen; this is preferable.
– In the last reaction, the pH should be >7, since the medium is supposed to be alkaline.
– Also, the spelling varies throughout the text: Benedict/Benedict’s/Benedicts’, Fehlings/Fehling’s/Fehling/Fehlings’.
Thank you so much, as always. Finally got around to fixing those items. Greatly appreciated!