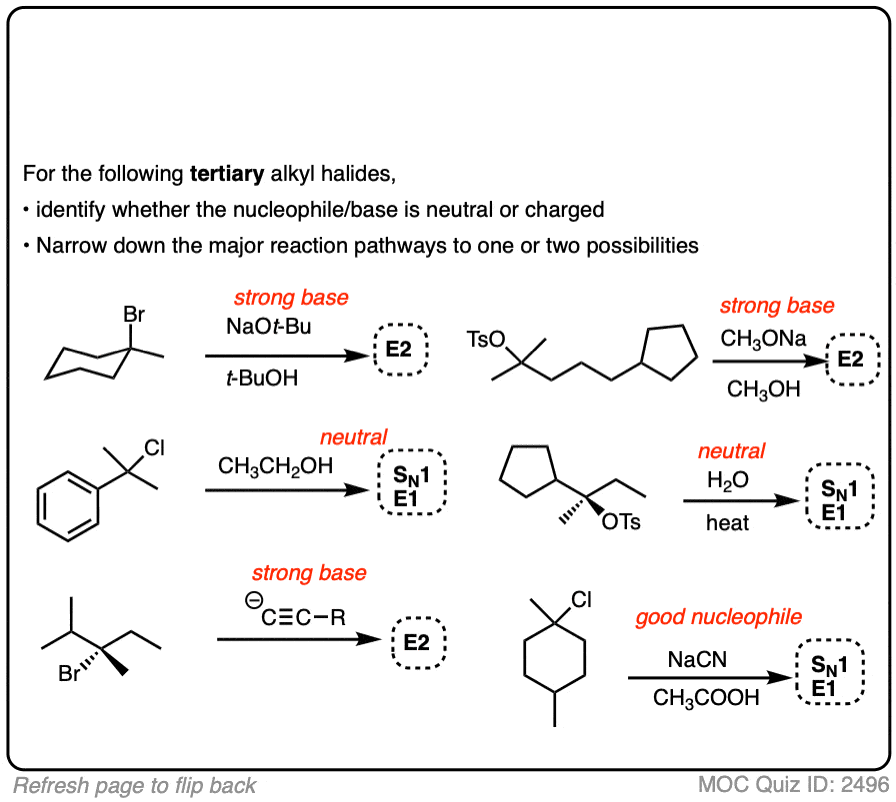
SN1/SN2/E1/E2 Decision
Deciding SN1/SN2/E1/E2 (2) – The Nucleophile/Base
Last updated: May 7th, 2025 |
SN1 / SN2 / E1 / E2 The Nucleophile / Base
- This article assumes you understand the mechanisms of the SN1/SN2/E1 and E2 reactions. For review, see here [SN1] [SN2] [E1] [E2]
- SN1/SN2/E1/E2 reactions tend to happen on alkyl halides [see Identifying Where Substitution and Elimination Reactions Happen]
- Determining whether the alkyl halide is primary, secondary, tertiary (or methyl) helps to narrow down the possibilities [See SN1/SN2/E1/E2 – The Substrate]
- Primary alkyl halides tend to go through SN2 reactions. Tertiary alkyl halides never go SN2.
- Identifying the substrate alone often isn’t enough to narrow down the available possibilities, so the next step is to examine the nucleophile / base. That’s the purpose of this article!
- For our purposes, “strong” nucleophiles/bases are negatively charged and “weak” nucleophiles/bases are neutral
- A good rule of thumb is to expect SN2/E2 with “strong‘ (i.e. negatively charged) nucleophiles/bases and expect SN1/E1 with neutral nucleophiles/bases
- So the main focus of this article is in distinguishing SN1/E1 from SN2/E2, although this article will also discuss some ways of distinguishing SN2 from E2 based on basicity.
- Many quizzes and examples below, including exceptions.
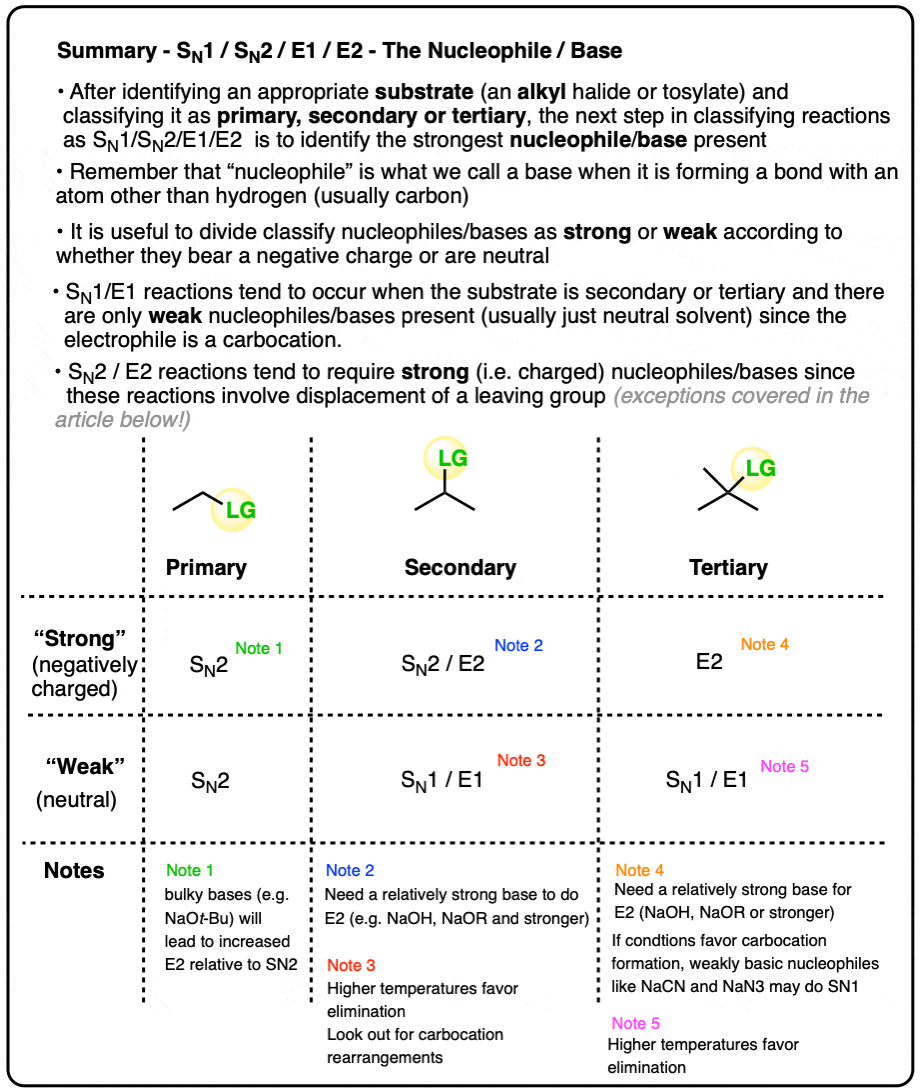
Table of Contents
-
- Identifying the Nucleophile / Base
- “Strong” (Negatively charged) Versus “Weak” (Neutral) Acids and Bases
- The Six Major Cases, Based On Substrate + Nucleophile/Base
- Secondary Alkyl Halides: The Importance of Base Strength
- More On The “SN1 vs E1″ and “SN2 vs E2″ Cases
- Do Acid-Base Reactions First
- Because You Can Never Have Enough Practice With Intramolecular Examples
- The Substrate Always Has The Final Say
- Summary
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Identifying the Nucleophile / Base
In previous articles were able to identify substrates as primary, secondary or tertiary. [See articles – Identifying Where Substitution and Elimination Reactions Happen, and SN1/SN2/E1/E2 – The Substrate]
This was helpful in being able to quickly rule out certain reactions.
- For example, if a substrate is primary, you can generally rule out the SN1 or E1 reactions since they go through a carbocation intermediate and primary carbocations are unstable. With few exceptions (bulky bases) primary substrates will generally go through SN2.
- If a substrate is tertiary, you can rule out SN2 since backside attack will be too slow due to steric hindrance impeding the nucleophile’s approach to the C-LG sigma* orbital
Once you have done this analysis, you’ll likely still have several possibilities. How can you narrow them down even further?
The next step in the deductive process is to examine the nucleophile or base. (Remember, a “nucleophile” is just what we call a base when it’s attacking an atom other than hydrogen, such as carbon.)
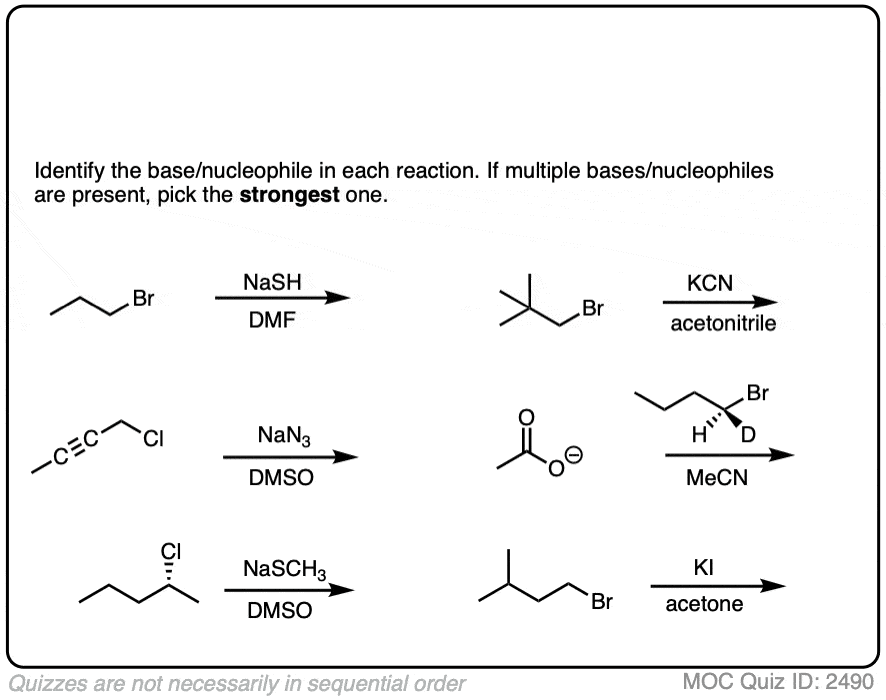
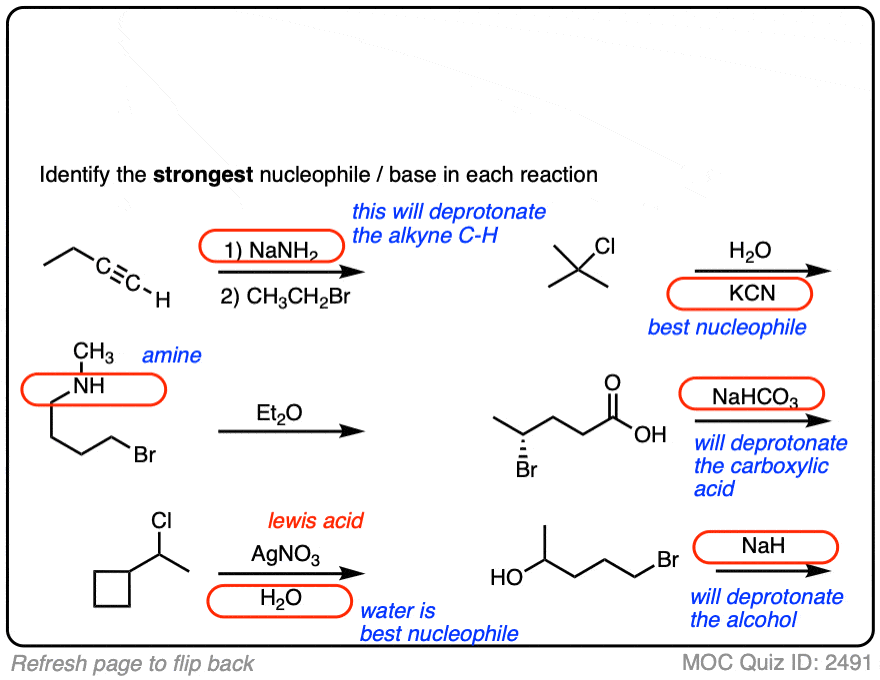
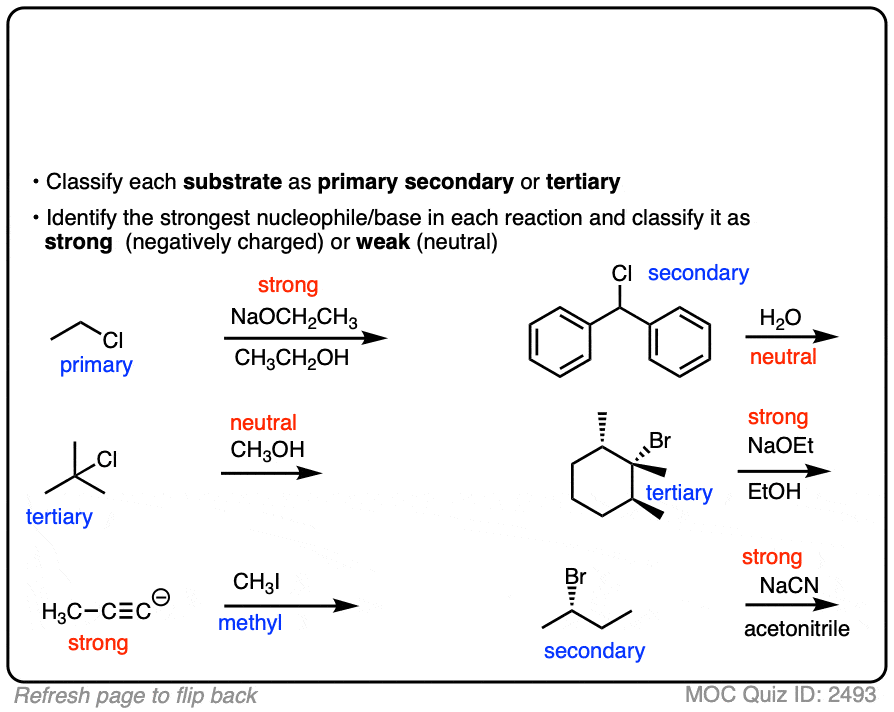
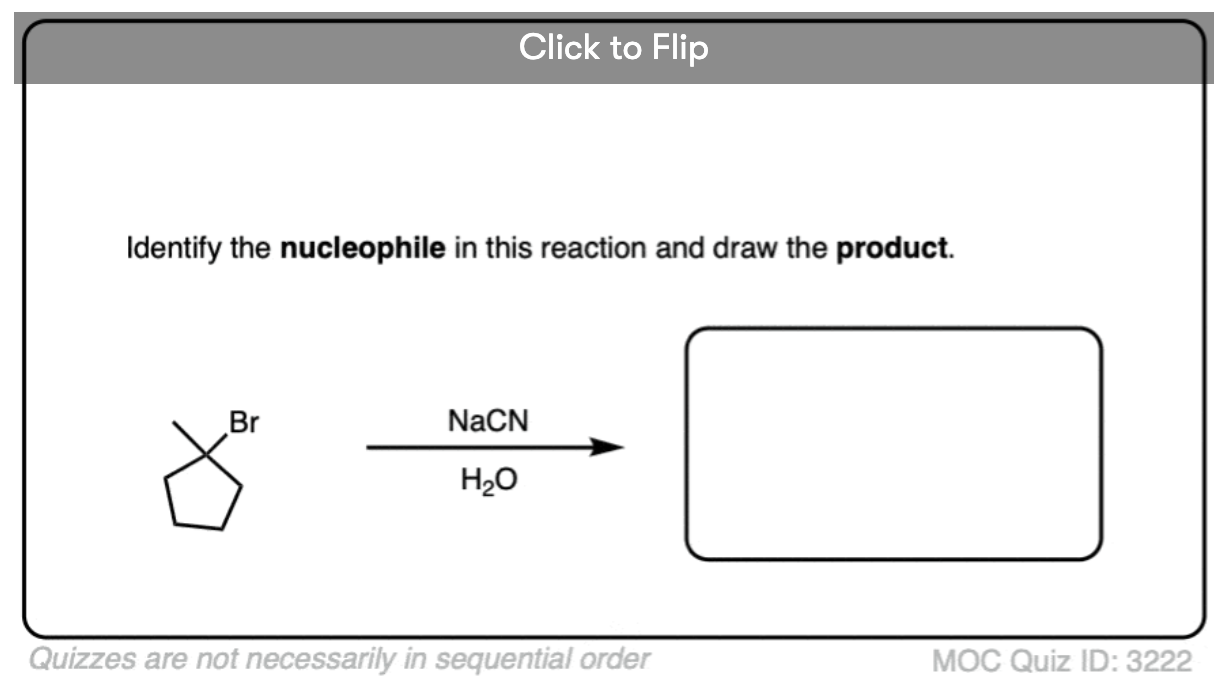
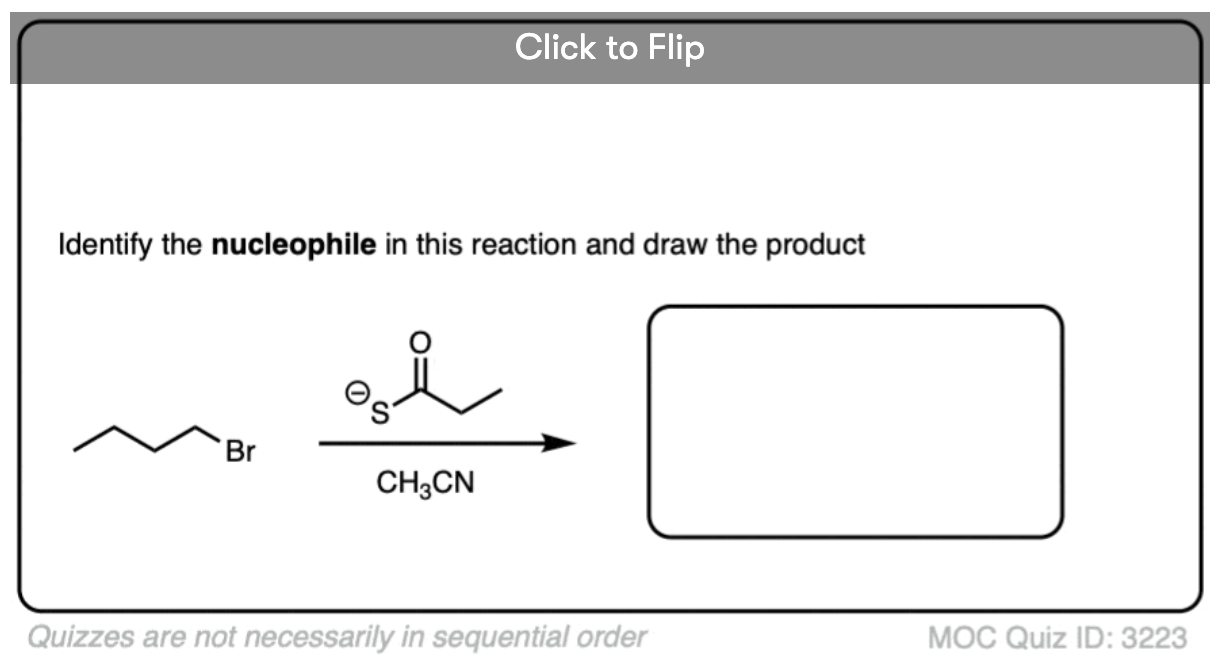
See if you can identify the nucleophile or base in these reactions below. When there are multiple potential nucleophiles/bases present, choose the strongest one. (More on that in a second).
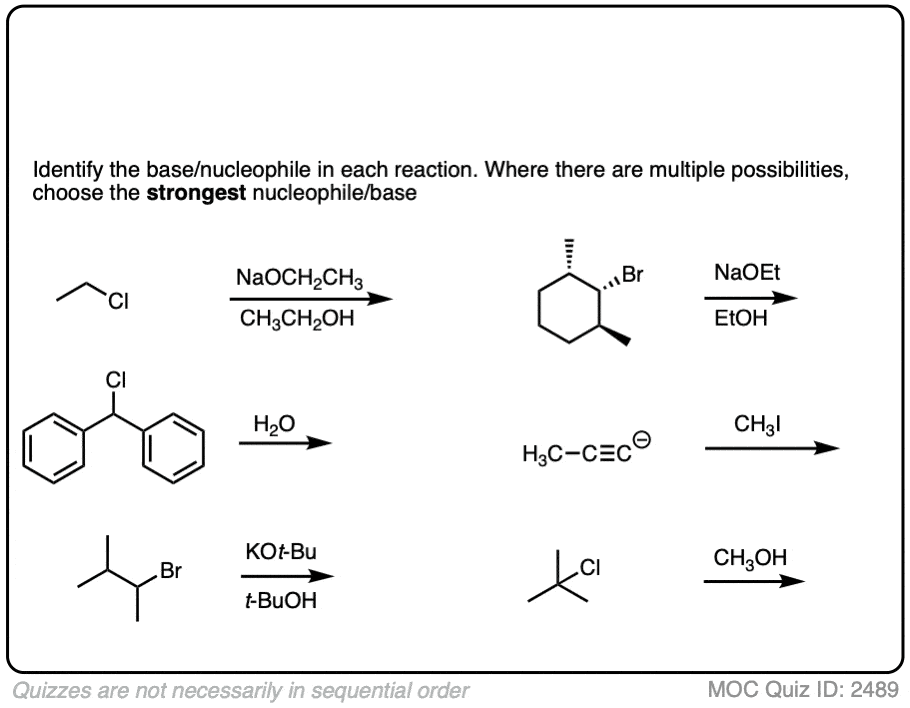 Click to Flip
Click to Flip
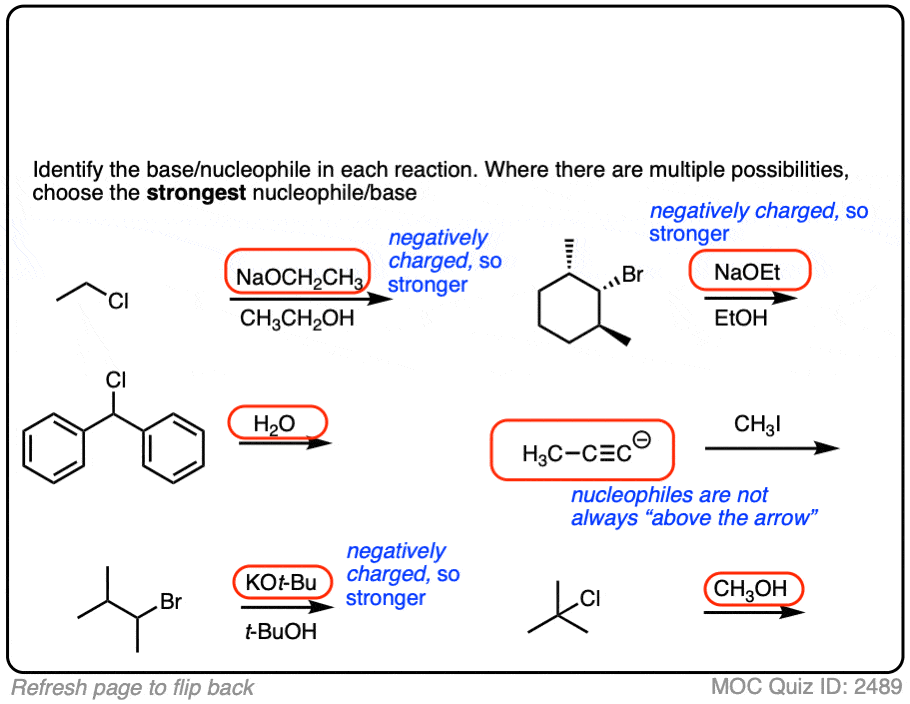
The substrate will react the fastest with the strongest nucleophile/base that is present.
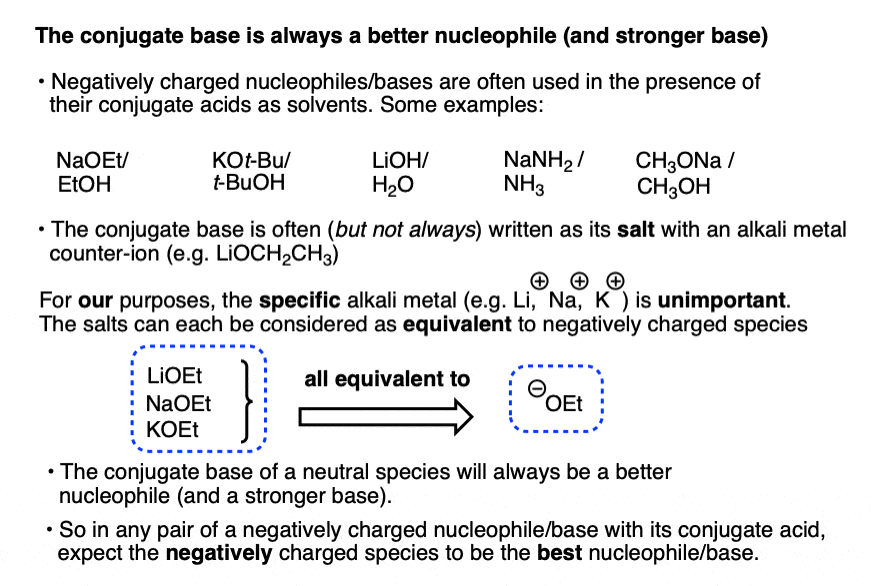
The conjugate base is always a better nucleophile [See article – What Makes A Good Nucleophile?], and the conjugate base is always a stronger base [See article – How To Use A pKa Table].
So if you see NaOCH2CH3 in the presence of CH3CH2OH or tBuO(-) in the presence of t-BuOH, the negatively charged species will be the active one.
Another tip: don’t fall into the common trap of assuming that the nucleophile will always be listed on the top of the arrow. Sometimes it’s actually the nucleophile that is drawn as the reactant and the substrate is over the arrow!
It’s better to try understand what’s going on than to try to memorize simplistic (and often faulty! ) rules like “the nucleophile will always be the first thing above the arrow”.
OK. Let’s look at another, slightly more complex set of questions.
 Click to Flip
Click to Flip
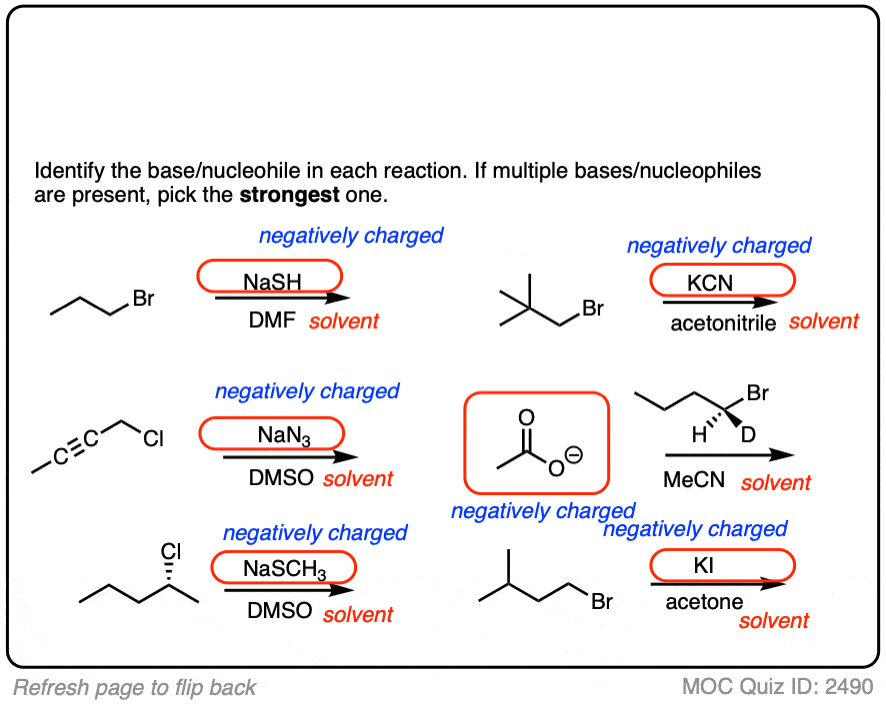
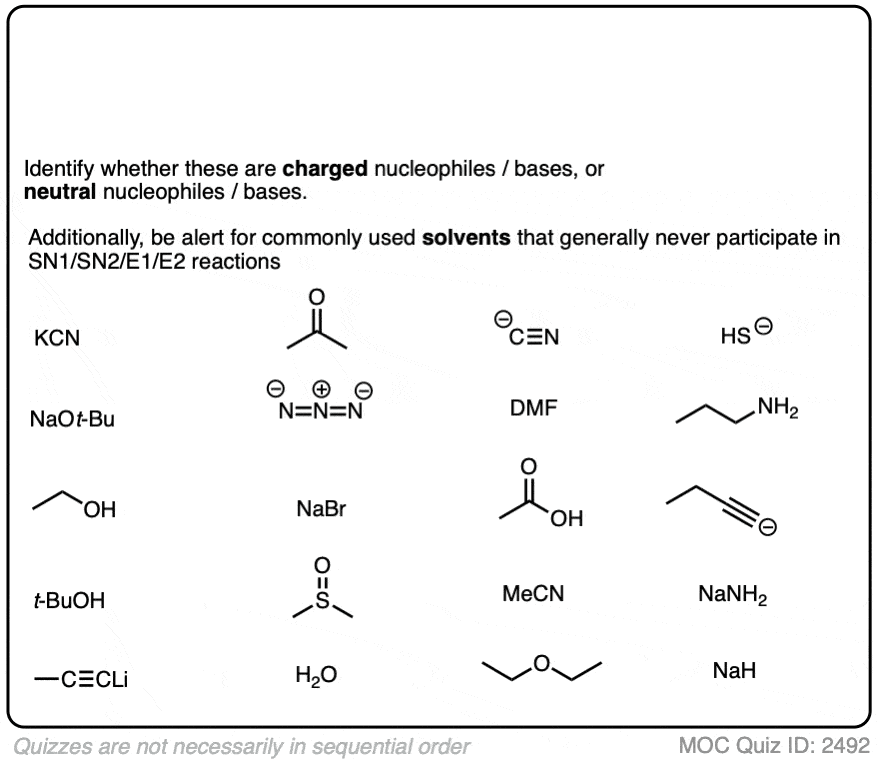
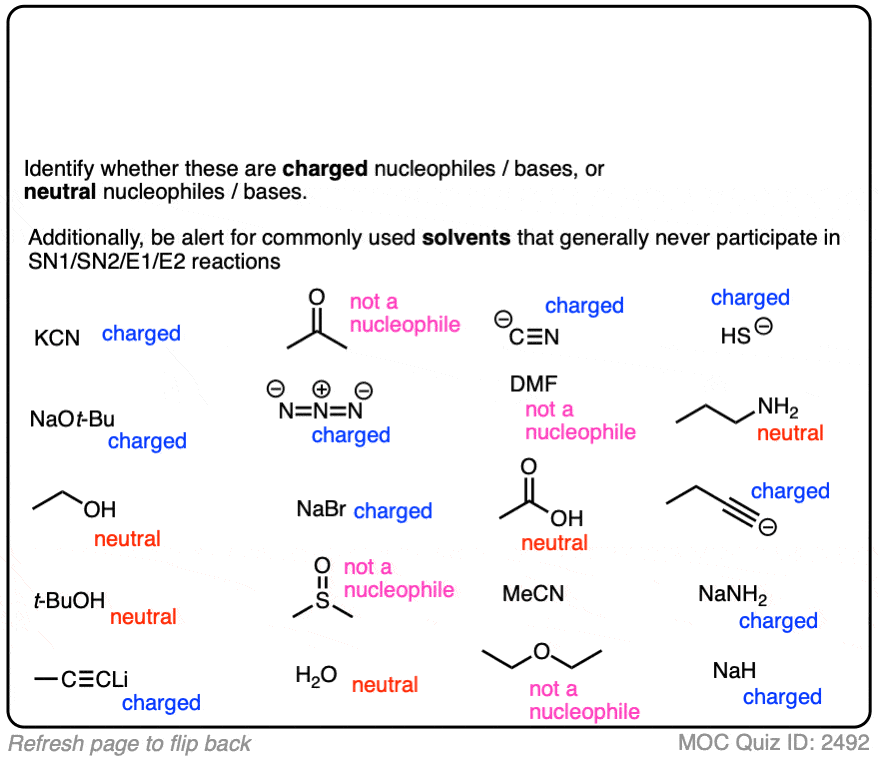
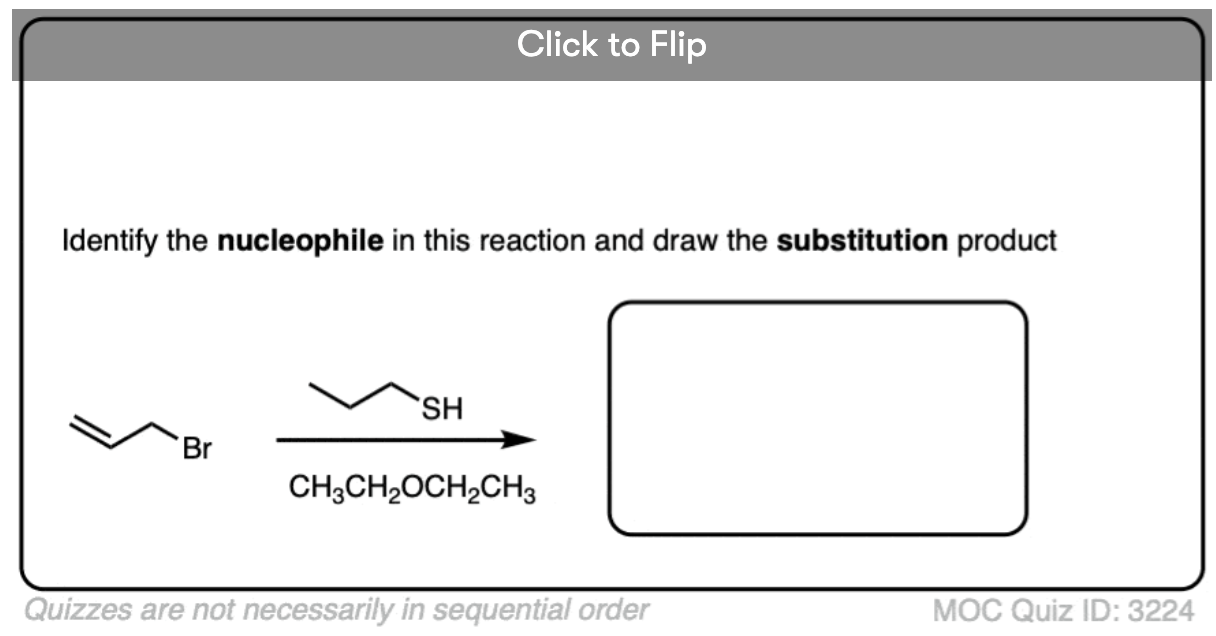
Another point which often trips students up is in failing to distinguish potential nucleophiles from various aprotic solvents which find use in, but do not participate in, these reactions.
Polar aprotic solvents are often chosen when substitution (usually SN2) is desired, since they are polar enough to dissolve charged nucleophiles, but cannot hydrogen-bond to them. The result is that nucleophiles in a polar aprotic solvent are relatively “free” and react faster with electrophiles, relative to their rates in polar protic solvents. [See article – Polar Protic? Polar Aprotic? All About Solvents]
Here are some common solvents to be aware of in substitution and elimination reactions. Note that they can be depicted in several different ways! (Acetonitrile, for example, can be written as CH3CN, MeCN or just “acetonitrile”.)
At the bottom are various polar protic solvents such as water, alcohols, carboxylic acids and even ammonia. These solvents are capable of participating in substitution/elimination reactions.
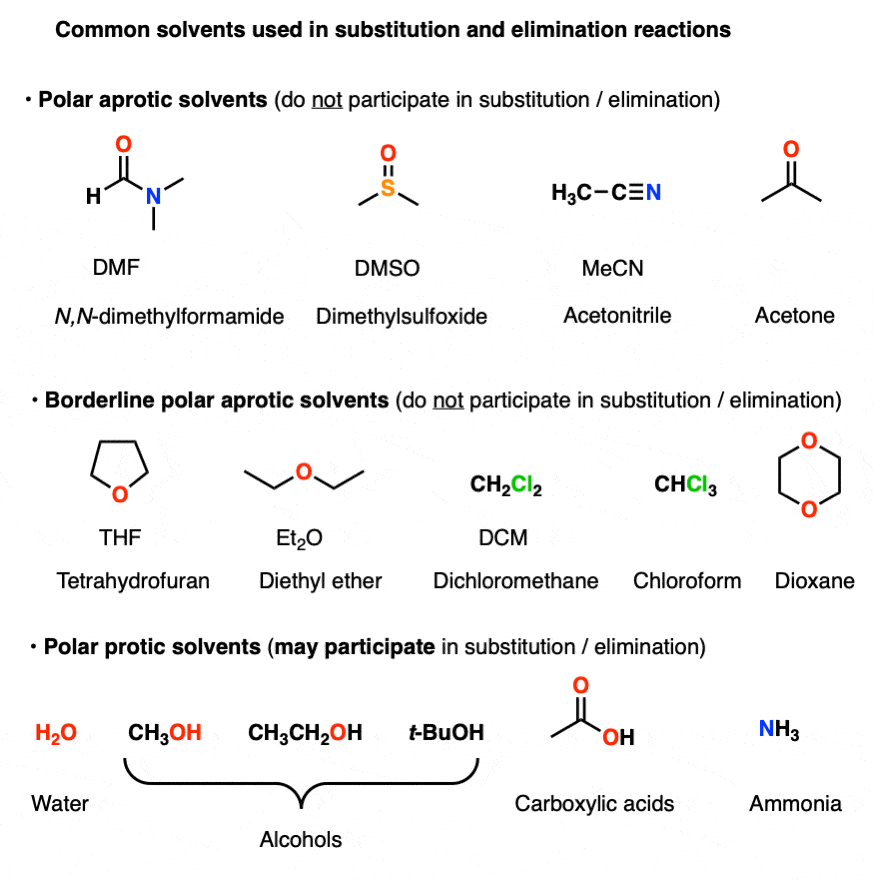
- Polar protic solvents such as water, alcohols, and even NH3 can potentially act as nucleophiles/bases in reactions.
- However, for our purposes, polar aprotic solvents will never be nucleophiles in SN1/SN2/E1/E2 reactions. [Note 1]
Note that the choice of solvent [polar protic vs polar aprotic] can have a strong influence on whether a reaction favors E2 or SN2. We’ll cover this in a subsequent article. [See article – Deciding SN1/SN2/E1/E2 – Solvent]
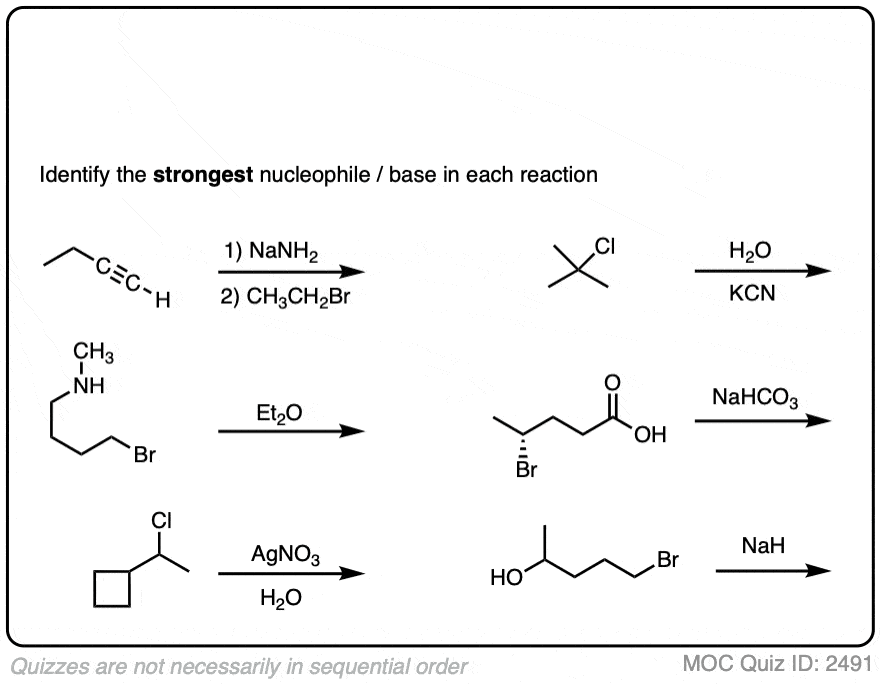
Here’s another set of more challenging cases:
 Click to Flip
Click to Flip
To summarize this section:
- There are no hard and fast rules about whether the nucleophile appears above or below the reaction arrow
- Look for negatively charged species to be your “best” nucleophiles/bases
- Know how to identify potential solvents – they will not participate in substitution or elimination, but they can influence the ratio of substitution vs. elimination products
- Sometimes the nucleophile / base is present on the substrate itself! These are examples of “intramolecular” reactions [More below]
2. “Strong” vs. “Weak” Nucleophiles / Bases
Now that we have had some practice in identifying the likely nucleophile, I am going to suggest a somewhat rough but very helpful classification that will help in distinguishing SN1/E1 reactions from SN2/E2 reactions.
Let’s call negatively charged nucleophiles “strong” and call neutral nucleophiles “weak“. [Note 2]
- SN2/E2 reactions tend to occur with strong nucleophiles/bases.
- SN1/E1 reactions tend to occur with weak nucleophiles/bases.
The rate determining step in SN1/E1 reactions is formation of a carbocation, which is generally only possible with secondary and tertiary substrates in highly polar, ionizing solvents like water, alcohols, carboxylic acids and mixtures thereof. Carbocations have an empty p-orbital and will readily combine even with weak Lewis bases such as water and alcohols since the carbon on the resulting product will have a full octet.
The rate determining step in SN2/E2 requires that the nucleophile/base displace a leaving group from a carbon that already has a full octet. Generally these reactions work best when the nucleophile/base is a stronger base than the leaving group.
Since alcohols, water, and carboxylic acids are relatively poor bases, many SN2/E2 reactions with them as nucleophiles/bases are disfavored from an acid-base standpoint. [See article: What Makes A Good Leaving Group] [Note 3]
With that in mind, classify the nucleophiles below as “strong” or “weak” (there are some land mines buried in this question!)
 Click to Flip
Click to Flip
3. The Six Major Cases, Based On Substrate + Nucleophile
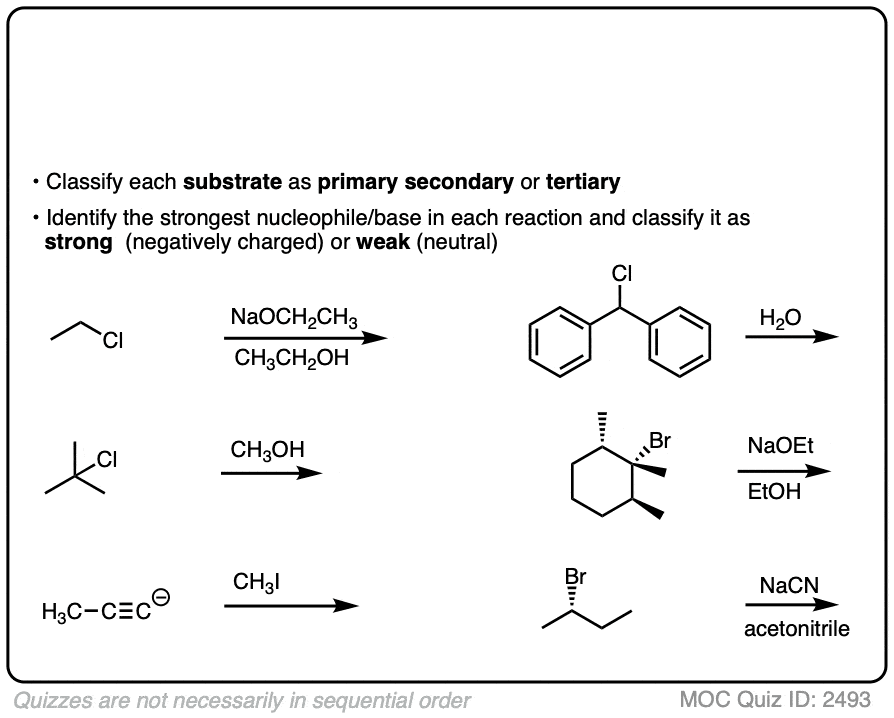
You have already learned how to identify substrate as primary, secondary, tertiary.
Having identified the best nucleophile, and classifying it as strong or weak, we’re now ready to start applying this to some reactions. See how you do:
 Click to Flip
Click to Flip
Since there are really only six major cases (not counting methyl) it might be helpful to draw up a grid like this:
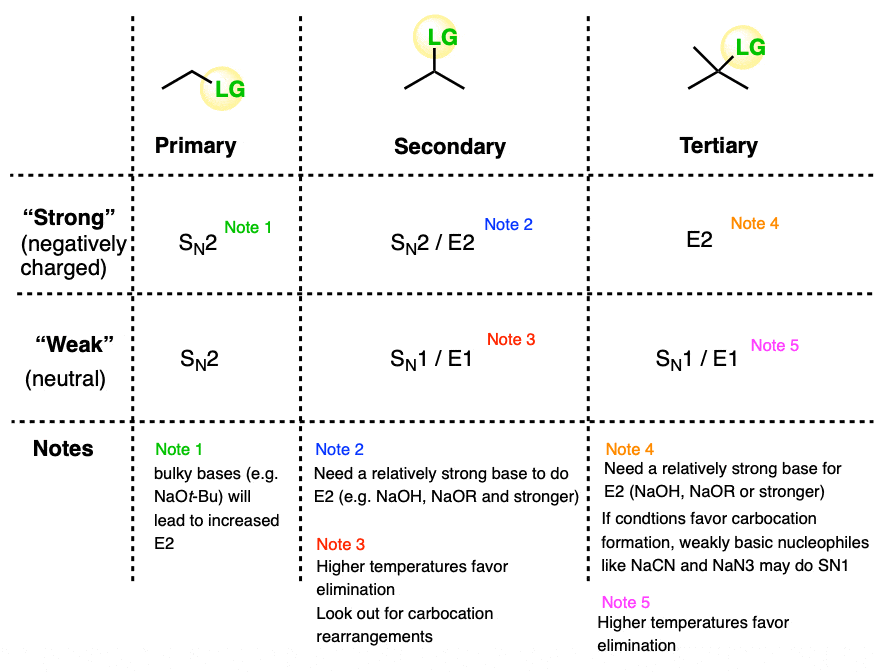
The six general cases are:
- Primary / strong and primary / weak . With rare exceptions (bulky bases like KOt-Bu) these will be SN2.
- Secondary / strong will be SN2 or E2
- Secondary / weak will be SN1 or E1
- Tertiary / strong will be E2 with only rare exceptions [Note 4]
- Tertiary / weak will be SN1/E1 [Note]
Here is some more practice:
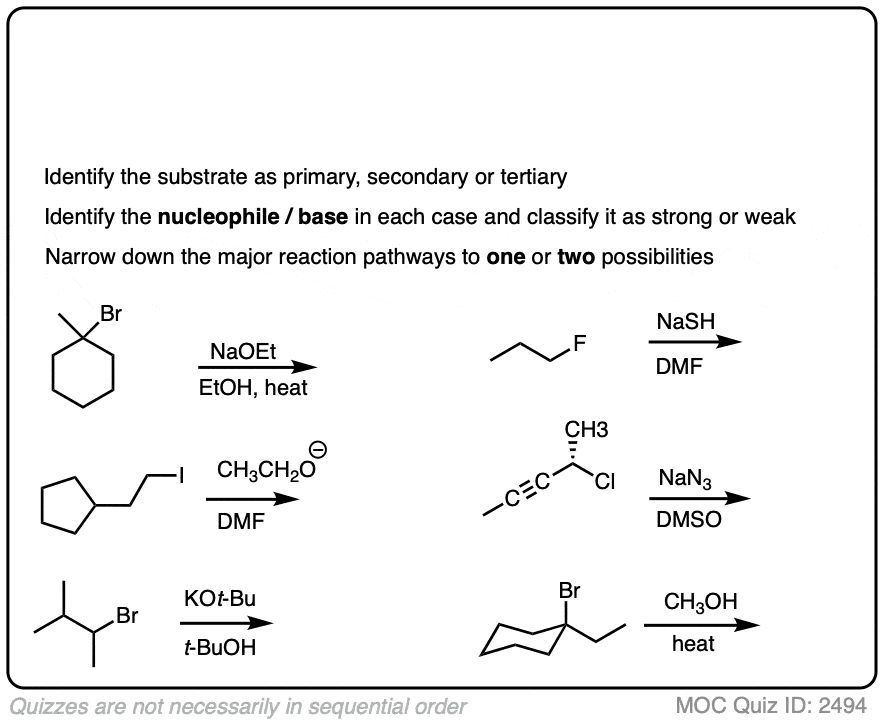 Click to Flip
Click to Flip
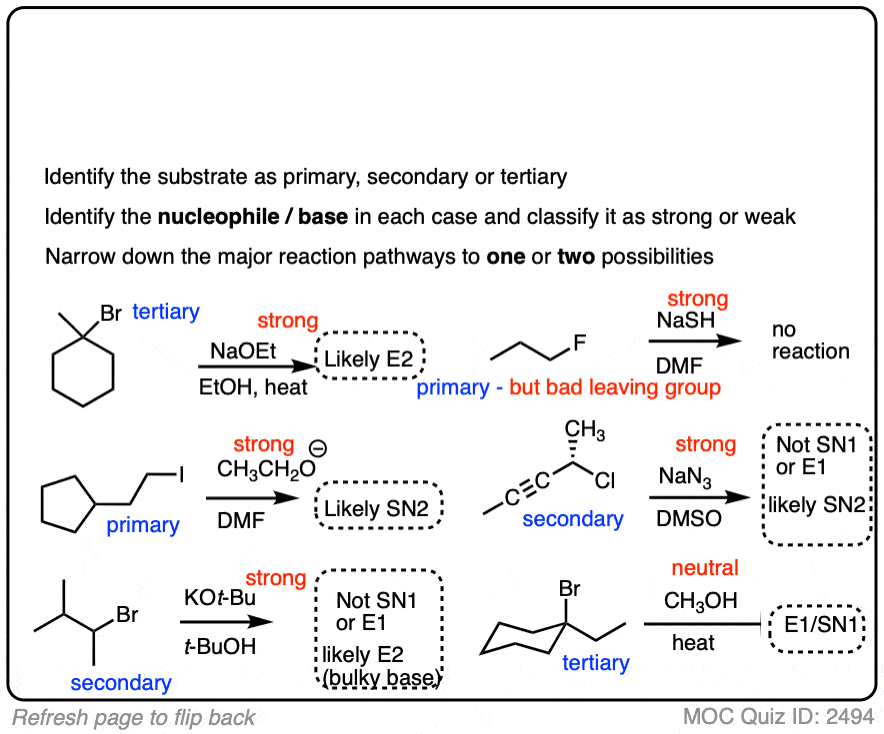
You might find it annoying that after all this work of analyzing the substrate and the nucleophile we still have multiple situations where we still can’t really nail down the dominant pathway.
Helpfully, analyzing the basicity of some strong nucleophiles/bases will help us make some clearer distinctions.
4. Secondary Alkyl Halides: The Importance of Base Strength
The E2 reaction generally requires a strong base. Negatively charged species that are weak bases such as halides, carboxylates, cyanide, azide, or thiolates [RS(-)] generally won’t perform E2 reactions. [Note 5]
Strong bases such as hydroxide ion HO (-), alkoxides RO(-), and species more basic than them (e.g. acetylides, amide bases, hydride) are fully capable of performing E2 reactions, however.
So a reasonably good dividing line for “E2-capable” bases is around a pKa of 12 for the conjugate acid. More basic than thiolates, in other words.
This chart might help. Note that this is specific for secondary alkyl halides.
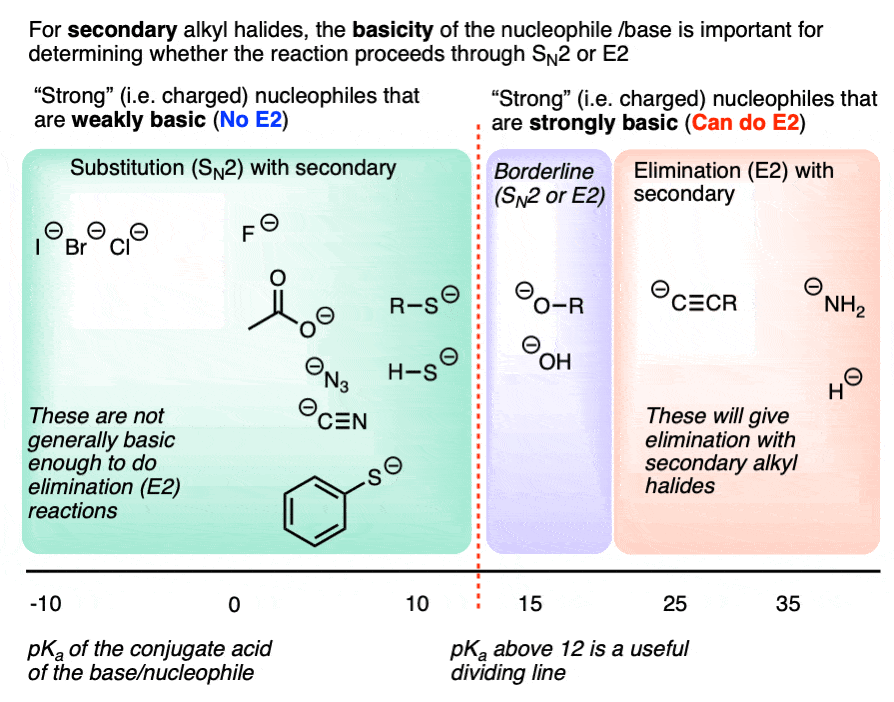
For secondary alkyl halides with weakly basic nucleophiles, expect SN2 for everything up to thiolates, and elimination for everything more basic than alkoxides. Amide bases (e.g. NH2(-), R2N(-), NaH, and acetylides will primarily give E2 products. (Acetylides are fine nucleophiles with primary alkyl halides, but will perform E2 reactions on secondary alkyl halides).
Depending on conditions, hydroxide and alkoxides themselves can go either way with secondary alkyl halides, We’ll cover this in more detail in a subsequent article. [See article – Deciding SN1/SN2/E1/E2 – The Solvent]
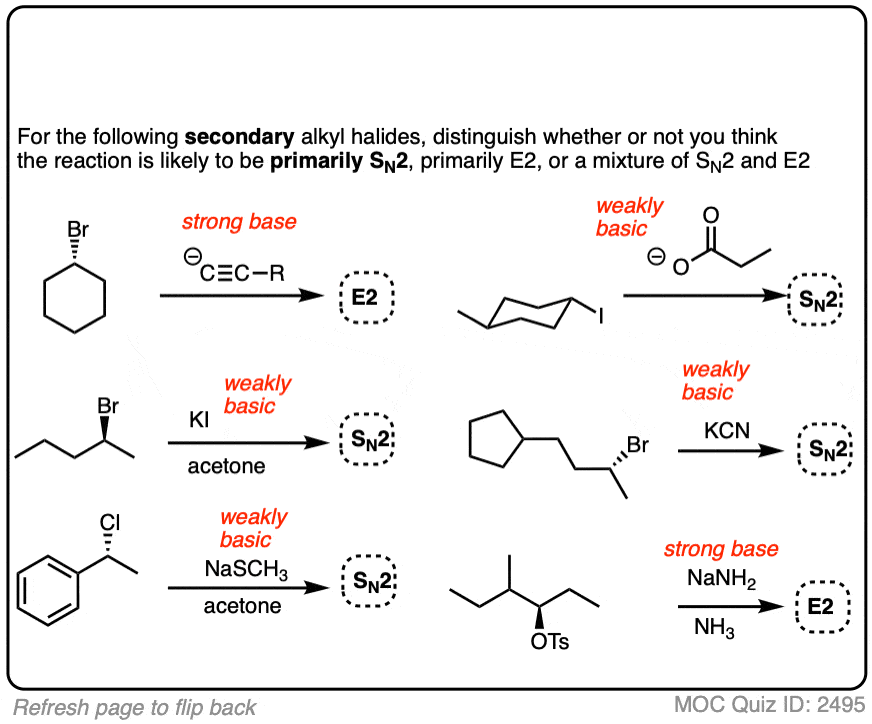
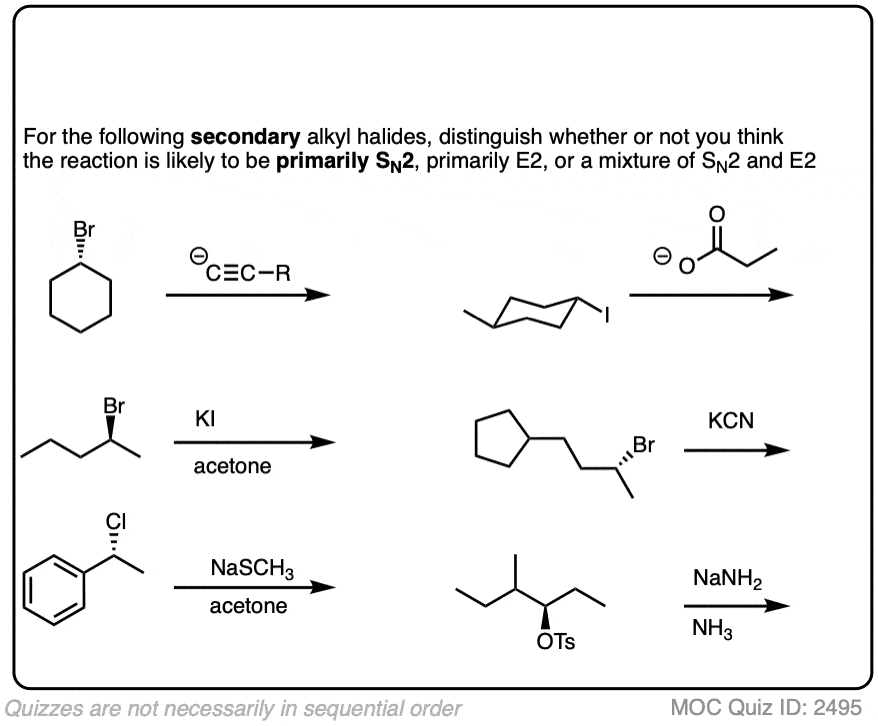 Click to Flip
Click to Flip
The E2 reaction on tertiary alkyl halides also requires a reasonably strong base such as hydroxide, alkoxides, or species more basic than that. (One exception – neutral nitrogen bases such as triethylamine or DBU will also perform E2 reactions)
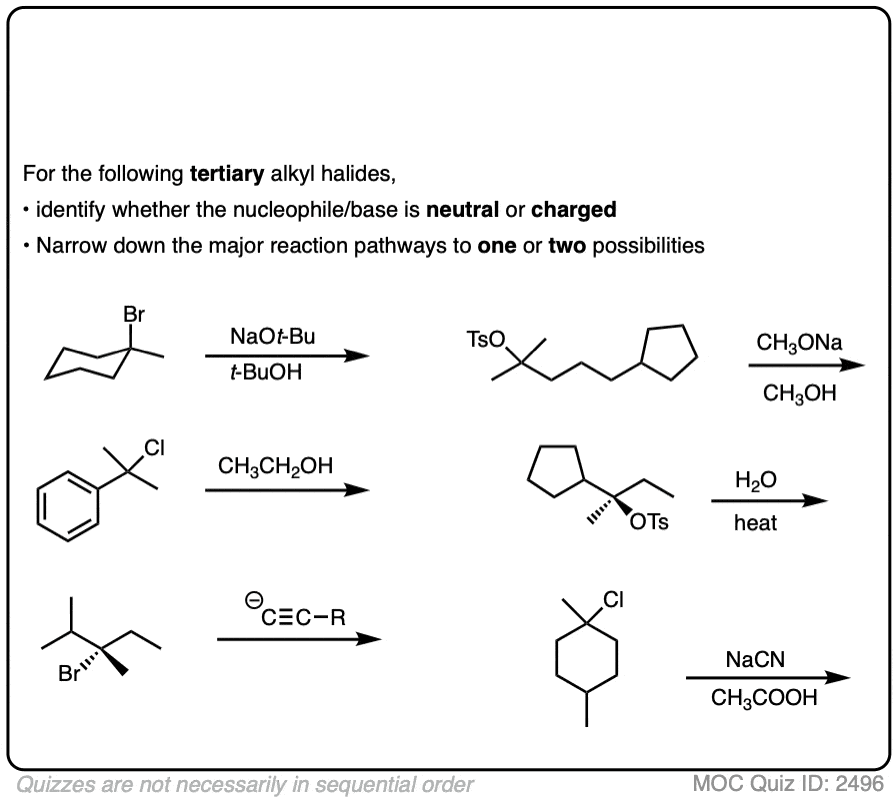 Click to Flip
Click to Flip
SN1 reactions are sometimes observed when “strong” , weakly basic nucleophiles such as (-)CN or N3(-) are in solution with a tertiary alkyl halide under conditions that favor carbocation formation. These species are such excellent nucleophiles that they can out-compete the solvent. [Ref ]
5. More on the “SN1 vs E1” and “SN2 vs E2″ Cases
At this point we have shown how to identify alkyl halides as primary, secondary, or tertiary, then how to identify nucleophiles as “strong” or “weak”, and then how to further narrow down negatively charged nucleophiles so as to identify which would be more likely to perform SN2 or E2.
The two problems that remain for a comprehensive assignment of SN1/SN2/E1/E2 are:
- distinguishing SN1 from E1 (the short answer is that heat favors elimination, more on that in a subsequent article – see Deciding SN1/SN2/E1/E2 – The Temperature).
- distinguishing SN2 from E2 for secondary alkyl halides with strong nucleophiles/bases in the “borderline” region [hydroxide and alkoxides]. (My short answer here – besides “ask your instructor, because many disagree” – is that polar aprotic solvents will favor substitution, but more in Deciding SN1/SN2/E1/E2 – The Solvent).
We will deal with these in subsequent articles.
Other than that, are there any other loose ends and exceptions to tie up? Yes!
Let’s finish up by dealing with acid-base reactions, intramolecular examples, and a final word from your substrate.
6. A Reminder To Do Acid-Base Reactions First
In our rush to cover the basics of strong and weak nucleophiles, above, we only considered that a nucleophile/base would either do substitution or elimination.
There is actually a third possibility (a fourth, if you count “no reaction”).
The nucleophile/base can also perform an acid-base reaction on the substrate.
This is most likely when reasonably acidic substrates such as alcohols, thiols, terminal acetylenes and carboxylic acids are treated with strong bases.
Acid-base reactions tend to be fast, relative to substitution and elimination reactions [See article – Acid-Base Reactions Are Fast]. So it makes sense to apply them first.
The resulting conjugate base will be, as we discussed above, a superior nucleophile/base relative to its conjugate acid.
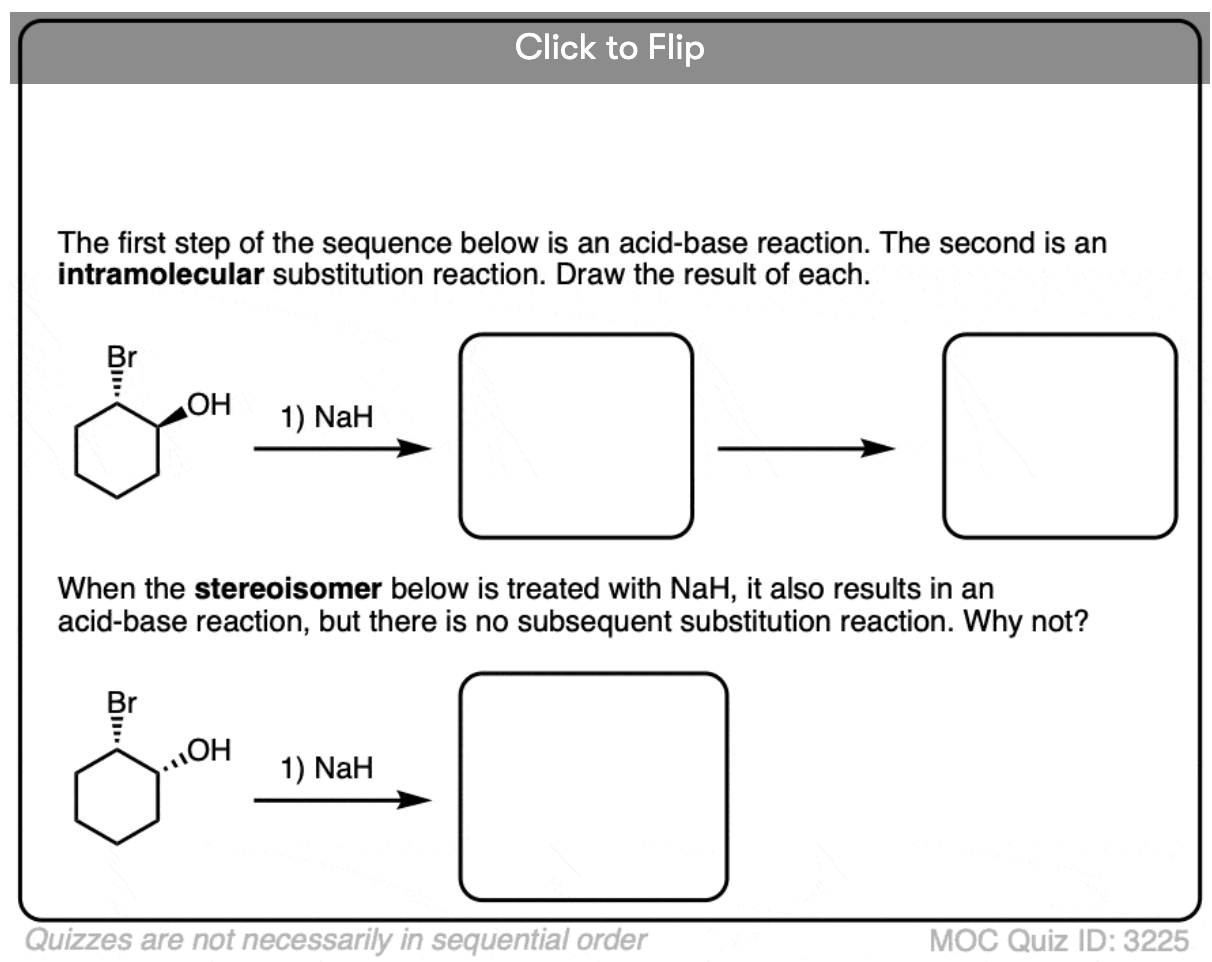
These reactions show examples of a deprotonation step followed by a substitution step. See if you can draw the products.
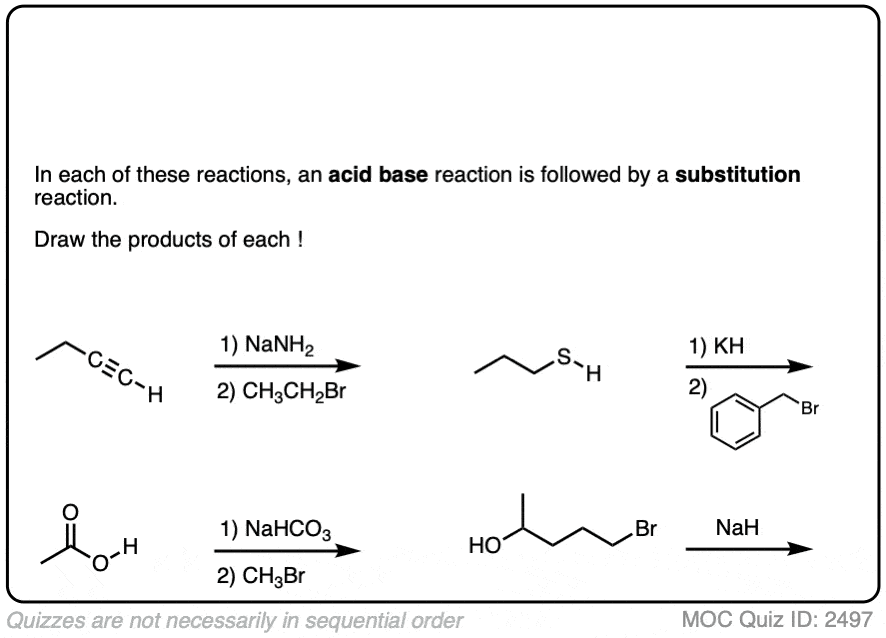 Click to Flip
Click to Flip
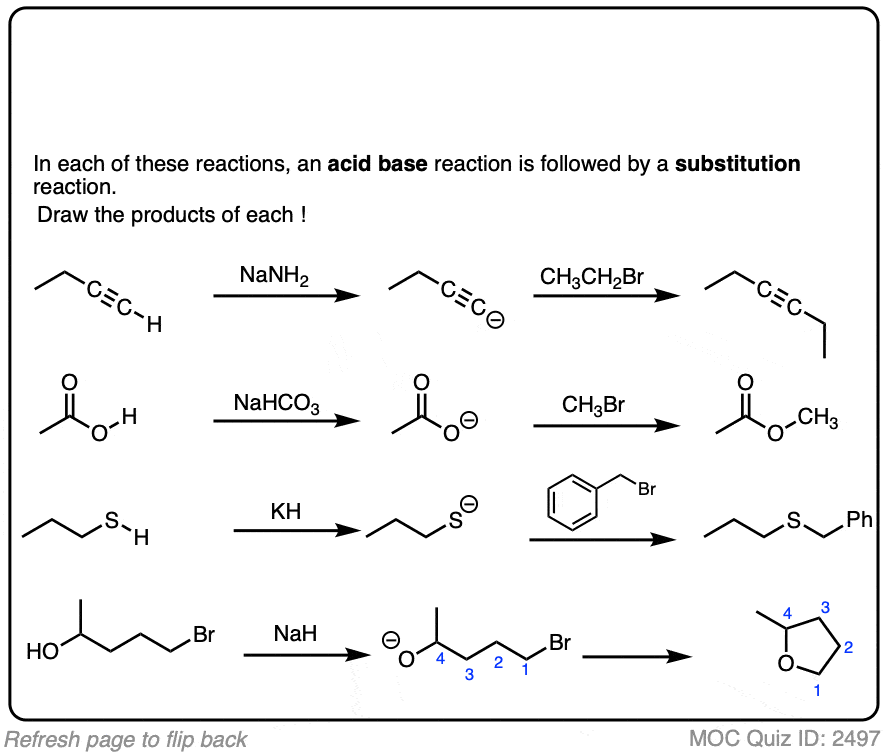
The corollary to “the conjugate base is a better nucleophile” is “the conjugate acid is a better leaving group”.
Addition of acids (including Lewis acids) will help turn poor leaving groups into better leaving groups.
This is most often seen with alcohols, where addition of H+ converts the poor -OH leaving group into the (much better) leaving group H2O.
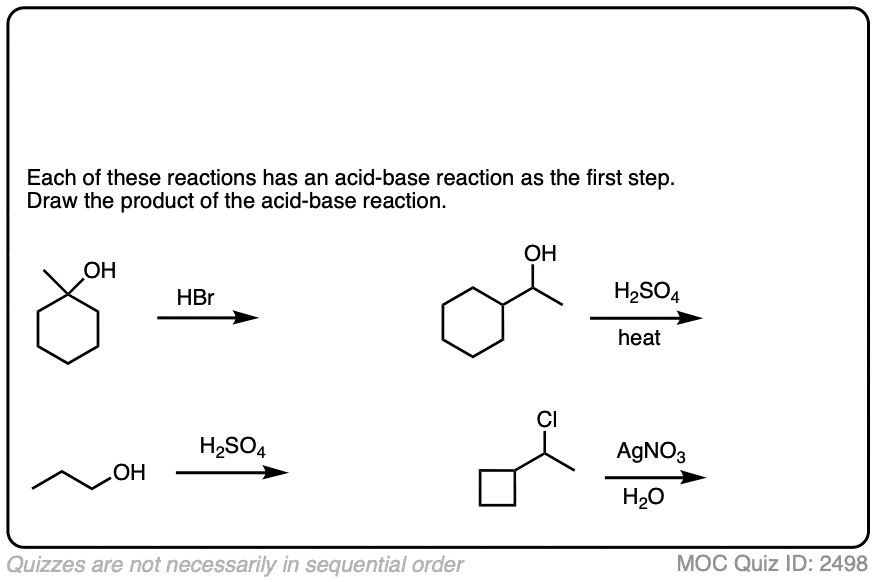 Click to Flip
Click to Flip
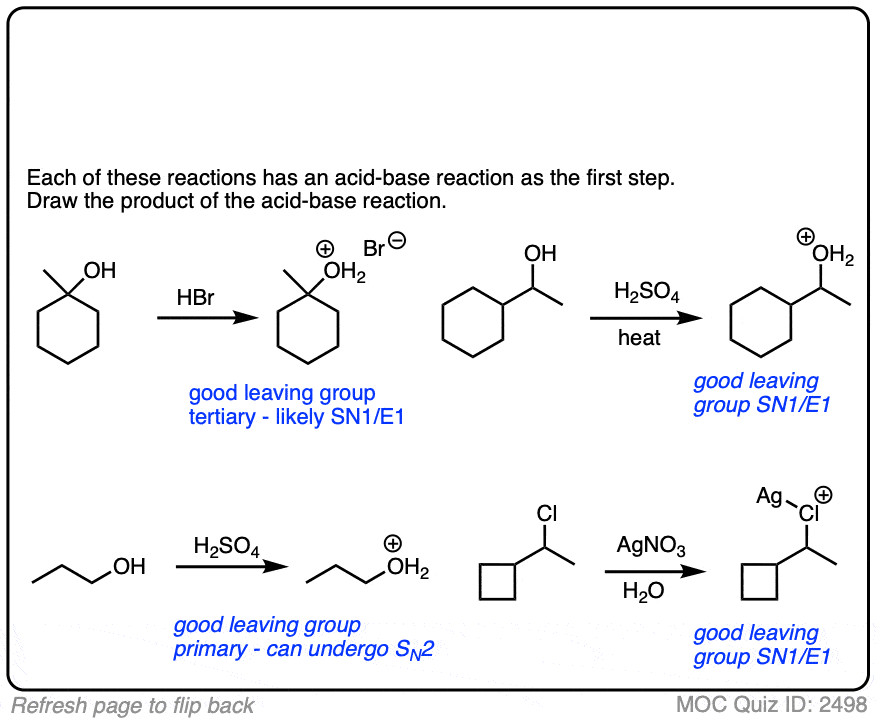
Halides can be made into better leaving groups through addition of silver salts, which irreversibly precipitate the halides out of solution.
7. Intramolecular Reactions
It always pays to look at the intramolecular variant of every new reaction you learn, since they are a perennial favorite of exam-writers everywhere.
In an intramolecular SN2 reaction, the nucleophile and substrate are on the same molecule. Their combination will result in a new ring.
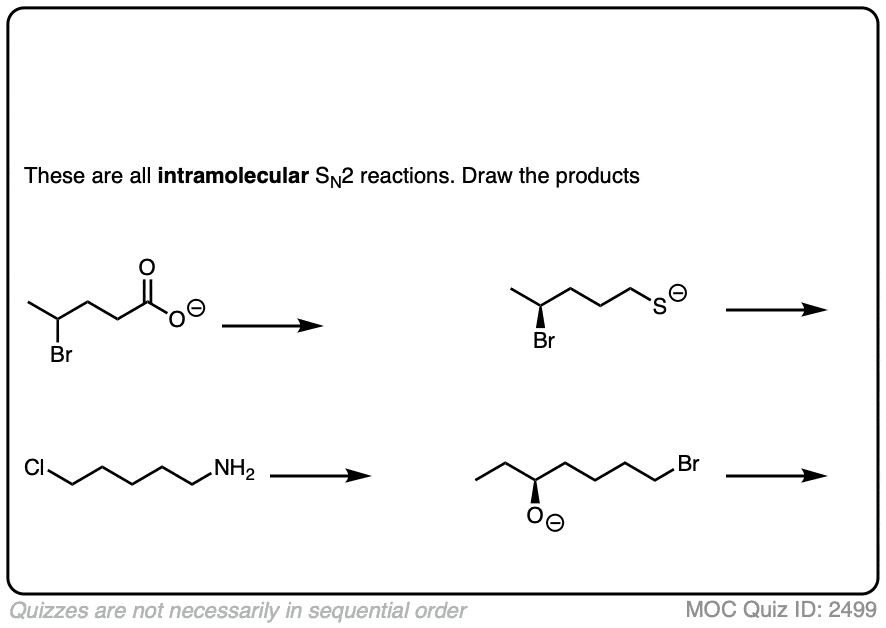 Click to Flip
Click to Flip
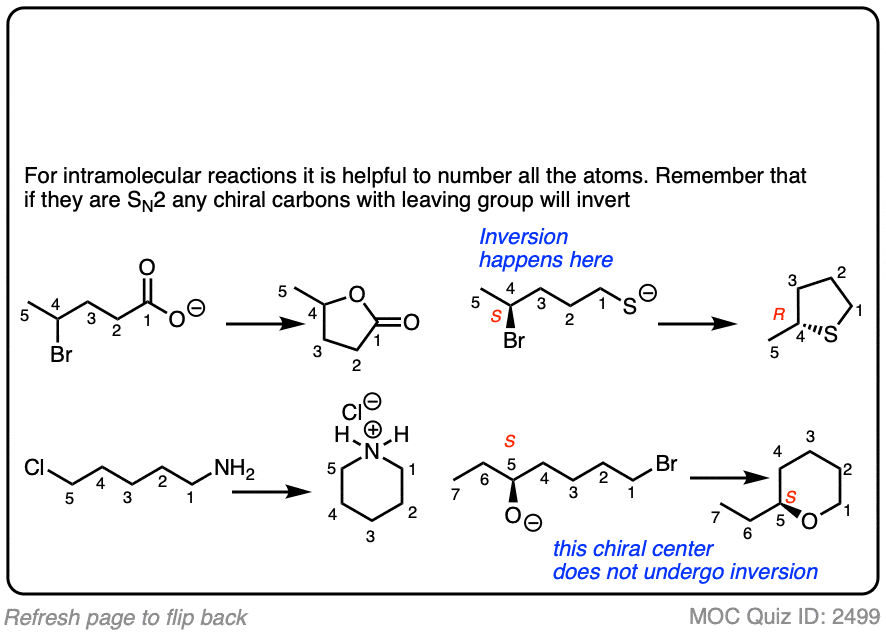
You can never have enough practice with intramolecular reactions.
8. The Substrate Always Has The Final Say
I know this is a lot to learn. Substrates, nucleophiles, reactions. That’s one of many reasons why students find organic chemistry difficult.
Rules of thumb, checklists, and flowcharts can be very helpful for making sense of all the information you’re asked to process.
You’re probably not going to like that there is one last thing, but there is one last thing.
At the end of the analysis process you will still have to apply the appropriate bond-forming/breaking pattern to your substrate in order to be able to draw the correct product.
And sometimes… even though you have run through all the checklists… your substrate might still have (or lack) a crucial feature that results in a specific reaction being unworkable.
Here are some examples.
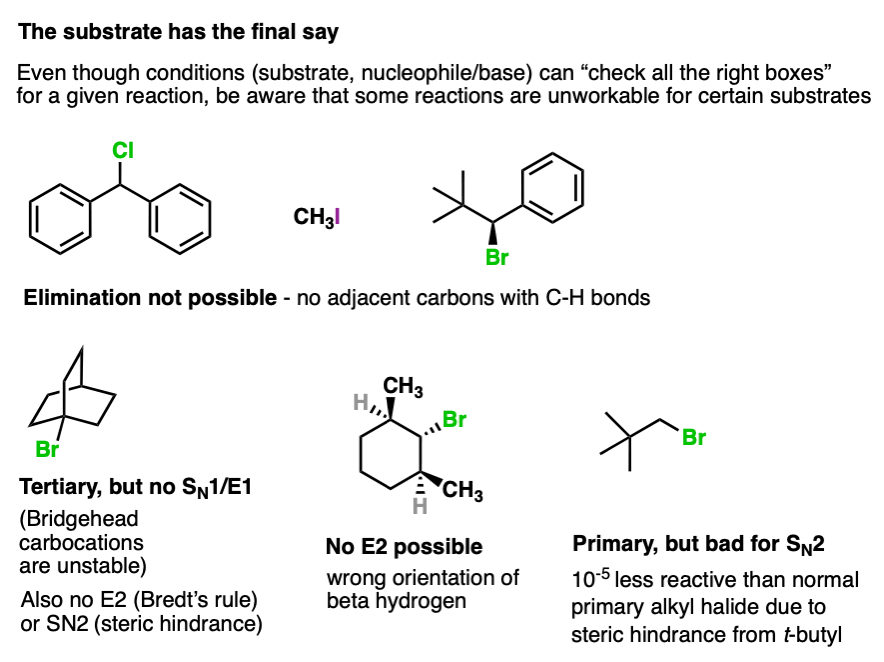
So by all means use flowcharts and rules of thumb, but don’t get so “locked in” on following a flowchart that you forget to actually look at the molecule itself and decide whether or not that reaction is even possible.
Your substrate always has a veto.
9. Summary
In the process of trying to decide if a reaction is SN1/SN2/E1/E2, there are five general steps. This article is the third of five.
We’ve previously covered Step 1 (look for alkyl halides [link]) and Step 2 (determine if the alkyl halide is primary, secondary or tertiary [link]). Step 4 (solvent [link]) and Step 5 (temperature [link]) are next.
In this article, we first looked at various reactions and looked for the strongest nucleophile/base present in each case. (A nucleophile is our name for a base when it’s forming a bond with any atom other than hydrogen).
- We then made a rule of thumb in defining nucleophiles/bases bearing a negative charge as “strong”, and neutral nucleophiles/bases as “weak”.
- This gives us six key cases to analyze (primary, secondary, tertiary with strong/weak).
- Primary alkyl halides tend to undergo SN2 reactions regardless of whether or not the nucleophile is strong or weak.
- E2 reactions generally require reasonably strong bases (as strong as hydroxide or alkoxides, or stronger).
- For secondary alkyl halides, strong nucleophiles/bases tend to perform SN2/E2 reactions, and weak nucleophiles/bases tend to perform SN1/E1 reactions.
- With secondary substrates, negatively charged nucleophiles that are weaker bases than hydroxide tend to do SN2 exclusively, and negatively charged nucleophiles that are stronger bases than hydroxides/alkoxides tend to do E2 exclusively.
- Hydroxide/alkoxide ions with secondary alkyl halides is a borderline area and may go either SN2 or E2. [More on that next]
- For tertiary alkyl halides, strong bases tend to perform E2 reactions, and weak nucleophiles/bases give SN1/E1 products.
- When acids/bases are added to the substrate, perform the acid-base reactions first.
- By all means apply rules of thumb / flowcharts, but still be alert for substrates where an SN1/SN2/E1/E2 reaction might not work due to the presence or absence of certain structural features (like the lack of a beta carbon with a C-H bond making E2 impossible for instance).
Notes
Note 1. The lone pair on acetonitrile (and other nitriles) will react with carbocations in a reaction known as the Ritter reaction but that’s beyond the scope of what we’ll cover here. [link]
Note 2. The biggest quibble I have about this “strong” and “weak” designation is that neutral amines such as triethylamine are perfectly capable of performing E2 reactions.
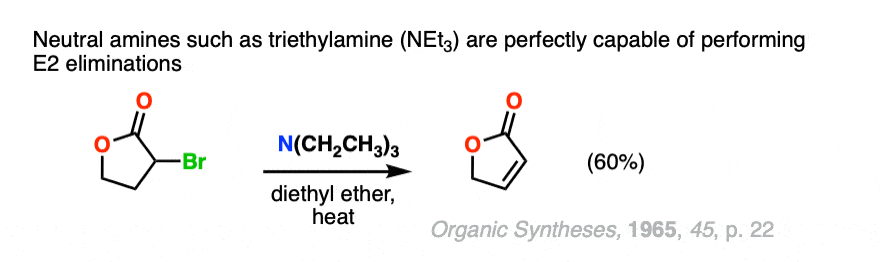
Other nitrogenous bases such as DBU and DBN will perform elimination reactions as well.
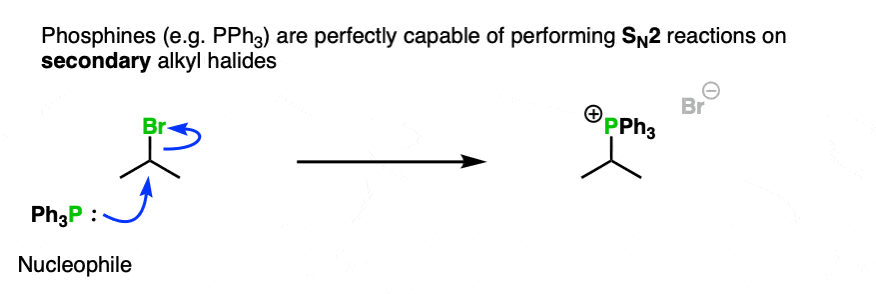
Secondly, neutral PPh3 is perfectly capable of performing SN2 on some secondary alkyl halides.
Note 3. You won’t go too wrong if you think of substitution reactions as being a fancy class of acid-base reaction, which tend to be successful when reaction of a stronger base with a substrate results in a weaker base (the leaving group).
One key difference is that substitution and elimination reactions tend to be irreversible and acid-base reactions are reversible.
Note 4. One example of a “strong” nucleophile/base reacting with a tertiary alkyl halide can be found in this example of an azide ion “intercepting” the carbocation formed from Ph3CCl in water.
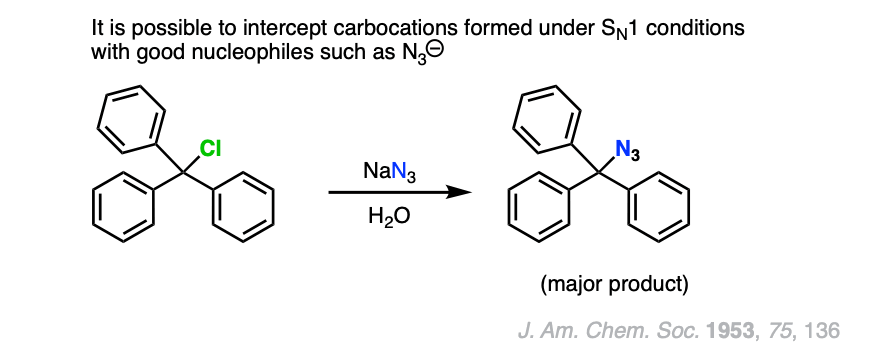
Note 5. Triethylamine and other neutral basic nitrogenous compounds are the main exception.
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
[literature]
Sir if we have 2-bromo-2-methylpropane and react with HCC-CH3 propyne in presence of NaNH2 . So will se get an E2 or a SN2 product as major product ?
The terminal alkyne will deprotonate first to make the acetylide. After that, the acetylide will deprotonate 2-bromo-2methylpropane to give an alkene (E2).
Primary alkyl halide + aq. KOH , product is decided by SN2 or E2 ?my teacher told it follow SN2 but according to quick and dirty method it will go for E2 ( as water is polar protic solvent ).Sir please tell the answer.I am very confused ?
Primary alkyl halides generally give SN2. The only exception would be something like neopentyl halides, which are very sterically hindered.
According to my textbook, why is it that when secondary alkyl halide tosylates react with alkoxides in DMSO, they form alkenes predominating (85%) over ethers? A polar aprotic solvent should favour Sn2 over E2, and tosylates are a very good leaving group. Shouldn’t this favour the nucleophile instead of the elimination? Thank you.
Your textbook actually does a better job of describing reality than most textbooks do. In reality the SN2 is pretty tough for secondary alkyl halides/ tosylates. May I ask what textbook you are using?
Thank you for this!!!
One question I have is when you say, “Here’s a good rule of thumb: if the conjugate acid of the base/nucleophile is less than 12” are you referring to pKa = 12 ?
Really, it’s a more quantitiative way of saying that hydroxide and alkoxides (pKa of conjugate acid around 14 and above) will tend to do eliminations, but thiolates (pka of conjugate acid around 10-11) will do substitutions.
Sir,
If i use sodium ethoxide in ethanol,on 2-chlorobutane what sort of pathway should the reaction follow…E2 or SN2?
I think it should be SN2 as the base is small and is a charged species so it can act as a nucleophile.
Also…
Does it make a difference if i use sodium ethoxide in different solvents for this reaction?
It’s a Williamson ether reaction.
Use ethanol, that’s fine.
Is sodium ethoxide a nucleophile? how can we come to know that?
It has a lone pair. Anything with a lone pair that can be donated (i.e. a Lewis Base) is potentially a nucleophile. When ethoxide attacks H, we say it’s acting as a “base” (specifically, a Bronsted base in this instance) which will result in elimination. When ethoxide attacks any atom other than H (like carbon) we say it’s acting as a “nucleophile”, which will result in substitution.
Would NaBr be an acceptable substitute for NaCl?
In what case?
From all of the practice i have since so many months, I guess the ‘Quick n Dirty’ rule for deciding SN1/SN2/E1/E2 is mostly (>95%) applicable, but whether it will perform substitution or elimination is more of a gut feeling. If you have done enough practice, you would mostly guess it correctly, purely due to experience.
Why is alcoholic KOH acts as base whereas aqueous KOH acts as nucleophile in E2 and SN2 reaction?
It completely depends on the substrate. What’s the reactant?
What is the use of CH3I & NANH2
I WAS GIVEN A RING 6 SIDES
OH AT ONE POSTION AT META POSTION OH AND CH3 AND AT ANOTHER META CH2OH
NaNH2 is a base. CH3I is an alkyl halide that reacts with alkoxides.
” Here’s a good rule of thumb: if the conjugate acid of the base/nucleophile is less than 12, an E2 reaction will be extremely unlikely. ”
I did not get the meaning of this. Please Explain.
This is a more precise way of saying that weak bases will not promote the E2 reaction.
in the case of aromatic amines, aniline, benzidine etc., how is the presence of a tertiary amine (triethylamine) induces nucleophilicity? (in aprotic polar solvent)
The pKa of aniline is about 30. Triethylamine is not a strong enough base to deprotonate it.
Hey can you tell me which mechanism aqueous NaOH would select
It really depends on the substrate.
Hi James,
Nice work. Just a quick question: In your example of ”Exception: Neutral Nucleophiles in SN2 and E2 Reactions” shouldn’t the triethylamine form the less substituted alkene. Here you show the most substituted alkene. The normal rule would be ”major product w/ bulky base: less substituted alkene formation (steric hindrance)” and I feel like NEt3 is pretty bulky. Can you explain? I have a hard time understanding this one.
You raise a valid point. This would likely result in two products, but I’m not sure of the ratio. I don’t think that NEt3 is so bulky that it would completely prevent formation of the more substituted alkene however.
Hi!!
I had a chem problem, so the original reaction is 1-bromopentane with NaOH which would undergo the SN2 reaction, however what experimental modifications can be done in order to form 1-pentene (E2 product). I already said a hindered base could be used like NaOC(CH3)3 however i need one more experimental modifcation, would heat work? Just by adding head to 1-bromopentane would that favour the E2 reaction rather than the SN2 reaction?
Yes, that sounds about right.
I see.
Do rates have factors at play here as well? Are SN2/E2 generally faster than SN1/E1 (second order > first order)?
Could you explain the reasoning behind rule #3? (having a charged nucleophile or base rules out E1/SN1)
Why wouldn’t a charged nucleophile attack a charged electrophile?
Generally, the E1 and SN1 occur with solvent as nucleophile or with very weak neutral nucleophiles. Thus when a charged species is added, it tends to be promoting an SN2 or E2.
Keep in mind these are rough guidelines. There are tons of exceptions – amines or phosphines as nucleophiles for example, or when a salt like KI is added to a tertiary alkyl halide such as t-butyl chloride. It’s more about how this subject is tested than anything else.
If our nucleophile is (CH3)3CONa, it is E2, right?
And the secondary reaction? SN1?
Yes, should be E2. That goes for secondary and tertiary alkyl halides.
You wrote…
“In both the SN2 and E2 pathways the reaction is “concerted” – that is, the nucleophile/base forms a bond as the C-LG bond is broken. Since there is significant bond-breaking occurring in the transition state, the energy barrier for this step is higher than in the case of the E1 or SN1”
But, the C-LG bond has to break even in the cases of SN1/E1 reactions… So how do the above lines explain anything?
You can think it this way: the carbon in an alkyl halide before SN1 is sp3 hybridised. Thus, it has tetrahedral Geometry. But after formation of carbocation, the carbon is sp2 hybridised and has planar geometry, hence , greater stability.
Ah… yes. I see how this is confusing. I was referring to the *second* step of the E1 and SN1 reaction, *after* the carbocation has formed. I edited the text to reflect that. Thank you.
P(Ph)3 is a weak base because the lone pair on Phospohorous is involved in resonance with the three phenyl groups and hence the lone pair is less available for donation .This would decrease its ability to act as a base (Lewis base concept).Firstly is this correct?
And since P(Ph)3 is a weak base it favours Sn2 over E2 even though its bulky.Right?
But isnt the conjugate base of a weak acid a strong base?HCN is a weak acid and hence its conjugate base CN- must be a strong base.
But it has been stated that CN- is a weak base and hence would not favour E2 over Sn2!
And I have a question.
What if the nucleophile/base is small but is a strong base…E2 would predomimate right?As the absraction of proton becomes easier?
CN- is a strong nucleophile but a weak base. It would favor substitution over elimination.
CN- is a weak base??!!how is that possible?
Quick question with regards to notation…when a reaction is written with one compound on the top of the arrow and one at the bottom, are those to be understood as the nucleophile (reagant) and solvent. Are they always written in this order? I realize this might be a very basic question, it’s been years since gen chem.
Thanks!
Generally speaking, yes. And yes, they are typically always written in this manner. A lot of the time, you will also see reaction conditions (like temperature and rxn time) written underneath the arrow as well.
if we had say 2-chloro-3-methylbutane and then reacted it with a weak nucleophile/base as in ethanol but we place it in a polar aprotic solvent such as acetone, would this mean we could any of the reactions, SN2, SN1, E1 or an E2 reaction because the polar aprotic solution favours SN2 and E2 reactions while because we have a weak base/nucleophile it should favour a SN1/E1 reaction? or is there something i’m missing, I initially thought because we have a weak base as in ethanol and a non bulky secondary alkyl halide within a polar aprotic solvent that would undergo a SN2 reaction, but then i realised that the ethanol base is not charged, should I assume it is charged by separating the ethyl R group and the OH, making [OH]-?
Ethanol by itself is unlikely to be basic enough to promote an E2 reaction; I would expect that an SN2 reaction with ethanol as the nucleophile and acetone as the solvent.
What happens if you have a starting product like Cl-CH3-CH3-CH3-CH3-OH and a nucleophile of OH- in DMF solvent? I know it doesn’t work, but why?
Aha… well, there’s a reaction besides substitution that can occur on Cl-CH2-CH2-CH2-CH2-OH (and it’s very fast compared to substitution). After that occurs, however, there IS a substitution reaction, although maybe not the one you might have expected
James how did you decide that the OH- will deprotonate the Cl-R-OH?
Does having the Cl makes the molecule more acidic than say an alcohol of the same length would be (which in turn makes it more likely to swap its proton with the hydroxide)?
If so doesn’t the length of the molecule significantly reduce the Cl’s effectiveness as an electron withdrawing group?
Generally acid-base reactions are much faster than substitution/elimination due to a rule we call “the rule of least motion” which means that proton transfers on atoms like O don’t involve hybridization changes or any other significant molecular reorganization, while sub and elim reactions on carbon do.
“Acid base reactions are fast”.
And why did you decide that this acid-base equilibrium:
OH- + CL-R-OH OH2 + CL-R-O-
would favor the products?
*arrows were deleted for some reason (equilibrium between CL-R-OH and OH2)
I think the best emergency shortcut, is if the substrate is tertiary you can rule out SN2 and if it’s Primary forget about SN1, It leaves you closer to the right answer and maybe some partial credit
Why comments are not seen??
Plz tell me the E2 reaction of CH3CH2CH{ S(CH3)2}CH2CH3 in the presence of reagent , according to Hoffman rule?
Start by drawing out the molecule. Your leaving group is S(CH3)2 and there should be a positive charge on the sulfur. You’re going to form a new pi bond by losing this leaving group and also a C-H bond on the carbon adjacent to the carbon bearing the sulfur. Try to draw out those potential products.
Dilek, when we talk about CH3COOH It’s ionize form Is CH3COO- And H , inwhich CH3COO- is a conjugated base and a good nucleophile bcoz it is higly negative and can donate electrones more efficiently..
What do you mean by “the conjugate base is always a stronger nucleophile?”
It means that OH-(conjugate base) is a stronger nucleophile than H20(acid). Whenever we form conjugate bases we get a negatively charged species. As negatively charged species is a better nucleophile we can call conjugate base as stronger nucleophile
Yes, exactly Akash.
“Here’s a good rule of thumb: if the conjugate acid of the base/nucleophile is less than 12, an E2 reaction will be extremely unlikely.”
Less than 12 what? I’m confused here! This tip sound very handy but I have no idea why figure 12 is supposed to stand for!
I don’t know if you have figured it out already, but I still just wanted to quickly respond… the figure ’12’ as given in the situation you mentioned is the numerical value for the pKa of a given compound. Basically, it tells you via a numerical measure the extent of the acidity and/or basicity of a chemical compound you are looking at. So, as James says, “if the conjugate acid of the base/nucleophile is less than 12,” then the base is probably too acidic and not enough basic to act as an eliminating agent for the E2 reaction pathway to occur for that set of reactants and reagents.
You should to able to find good reference tables with pKa values calculated and already listed for organic compounds and the like, either online or in a major chemistry textbook (especially o-chem textbooks), if you need to look up values! Hope this helps!
SORRY BUT WE DONT HAVE PKA VALUES IN EXAMS. WHY DOESNT ANYONE UNDERSTAND THIS. THAT IS WHY WE ARE ASKING HOW TO FIGURE THIS OUT–WE ARE NOT GIVEN THE PKA VALUES. THIS IS THE HARDEST PART, NUCLEOPHILES AND BASES CAN SOD OFF
You should honestly ALL be familiar with the pKa’s of the common functional groups, as well as the common acids. If any of you are planning to go beyond Organic I & II, you will definitely be REQUIRED to memorize these. They WILL show up on every exam you take, and you will need to know them so you’ll be able to work through a problem in a timely manner. After all, a lot of people perform poorly due to time constraints, especially the ones who DON’T memorize important pieces of information like pKa values and as a results, have to consult a textbook. If you start now, I guarantee they’ll be helpful and you won’t forget them.
Hey.Great site-I can understand and enjoy organic chem better now.
A Question.How can triphenyl phosphine be a good nucleophile ?? It has three phenyl rings then it should be sterically hindered.
First of all the PPh3 has a trigonal pyramidal geometry, so a lone pair is pointing in the opposite direction from the phenyl groups. Secondly, the C-P bonds are fairly long which decreases the steric hindrance somewhat. PPh3 is a good enough nucleophile to do SN2 reactions of primary and secondary alkyl halides.
So, on my chemistry homework I have the following equation:
CH3CH2CH3CH3OCH3 + N3- ——–> ?
with a solvent of acetone.
Is this an SN1, SN2, E1, or E2 reaction? I think it might be an elimination 2 reaction but I am confused because in this case we are not removing a halite and N3- isn’t anything I have listed for a base/nucleophile in my notes.
Do you see a good leaving group…………? that’s always the first question to ask
Underneath where you wrote, “In contrast, the bulky base below (tert-butoxide ion) is a strong base but a poor nucleophile due to its great steric hindrance, so an E2 reaction is much more likely than SN2.”
I think you have the wrong picture
thanks for this extremely helpful website though!
CH3OH is more acidic than water. The +I effect of CH3 group decreases the polarity of
O-H bond. What makes CH3OH more acidic.?
In Q2, part 3, Why is H2SO4 a neutral nucleophile? It seems like it would donate H+, and then become charged. HSO4- which is inert as a base (since it’s the conjugate base of a strong acid), but due to its negative charge could be a decent nucleophile.
The negative charge on oxygen in HSO4- is very resonance stabilized and simply does not act as a competent nucleophile in the vast majority of situations, so it’s easier to treat it as a non-nucleophile.
So then from where does the formation of hydrogen sulphonate come into the picture?
If you use H2SO4 as an acid with an alcohol, HSO4- is the counterion. It doesn’t typically react with the substrate.
regarding NR3,it is nucleophilic & is comparable with OH- due to the reason its lower electronegativity and inductive effect?
in the exception column PPh3 undergoes SN2 but P is stabilized by 3 Ph and therefore will undergo Sn1 right?or is e- density on P high as it is not electronegative(unlike F) even after resonance stabilization?
CH3OH is acidic than water and for that matter any alcohol ROH is in equilibrium with RO- and H+,so wont RO- attack as a good nucleophile ?I know it is a weird question yet still how do we explain it will only ROH attacking always and not RO-.Pls reply ASAP
Equilibrium for self ionization of alcohol ROH to RO- and H+ is disfavored by 10 to the 16th power.
but when we take reactions and see like weak base and weak acid,there is a formation of salt always.My point is if it starts the reaction then equilibrium shifts and whole of it would react!
Hi, what if you have a strong nucleophile and a strong base like OH- and you are reacting with a tertiary haloalkane. Does not Sn1 and E2 pathway is both possible? How would you then choose whether Sn1 work? Assume there is no solvent. Thanks!
E2 takes place as Oh- is a good nucleophile ie unstable so whatever comes into its hand it will take it moreover in Sn1 it needs to penetrate the steric hindrances provided therefore E2 is preferred.E2 doesnt need a polar aprotic solvent(we just say like that)
Why E1 not takes place
If the alkyl halides are primary, they’ll react at SN2 with moderate base, like -OH, -CN, or CH3O-.
Thank’s for share.
I had a tricky question on a test yesterday. It wanted to know if methylchloride reacting with H2O would be SN1 or SN2. The professor put it would be SN1, but wouldn’t the methylhalide force it to be SN2? I think this problem could do both or not react at all. What are your opinions? I apparently got it wrong because I picked SN2 since those were the only two options.
Tammy, I agree, it’s not a fair question.
Methyl halide would not react using SN1 because it’s impossible for a methyl carbocation to form. It would also not react using SN2 because H20 is polar protic.
Talk to your professor about it!
You got it wrong because you picked SN2 for methyl chloride???? Whoever is teaching you SN1 happens with methyl cations doesn’t know what they’re doing.
It has to be SN2. The methyl cabocation is extremely unstable. If it were a tertiary carbocation, it’d be stabilized by inductive effect. SN1 needs a stable cation to proceed. SN2 on the other hand doesn’t need one, and it directly attacks the electron deficient carbon atom forming an intermediate. The only thing putting out sn2 is steric hindrance, totally absent in a tiny carbocation like methyl. Yeah, it’s Sn2. At least you know better than the one who teaches you :D