Amines
Nucleophilicity of Amines
Last updated: September 20th, 2022 |
Nucleophilicity Of Amines
The relative nucleophilicity of amines doesn’t get a lot of coverage in many organic chemistry courses, but if we’re going to cover amines, it seems worthwhile to at least devote one post to their nucleophilicity trends.
Most of what follows shouldn’t come as a great surprise, as it will echo a lot of concepts and themes that have made previous appearances in the course (Org 1 in particular).
There are, however, a few interesting wrinkles which might help to put some of these concepts into a broader focus.
The most important difference between the basicity of amines and the nucleophilicity of amines is the sensitivity of nucleophilicity to steric effects.
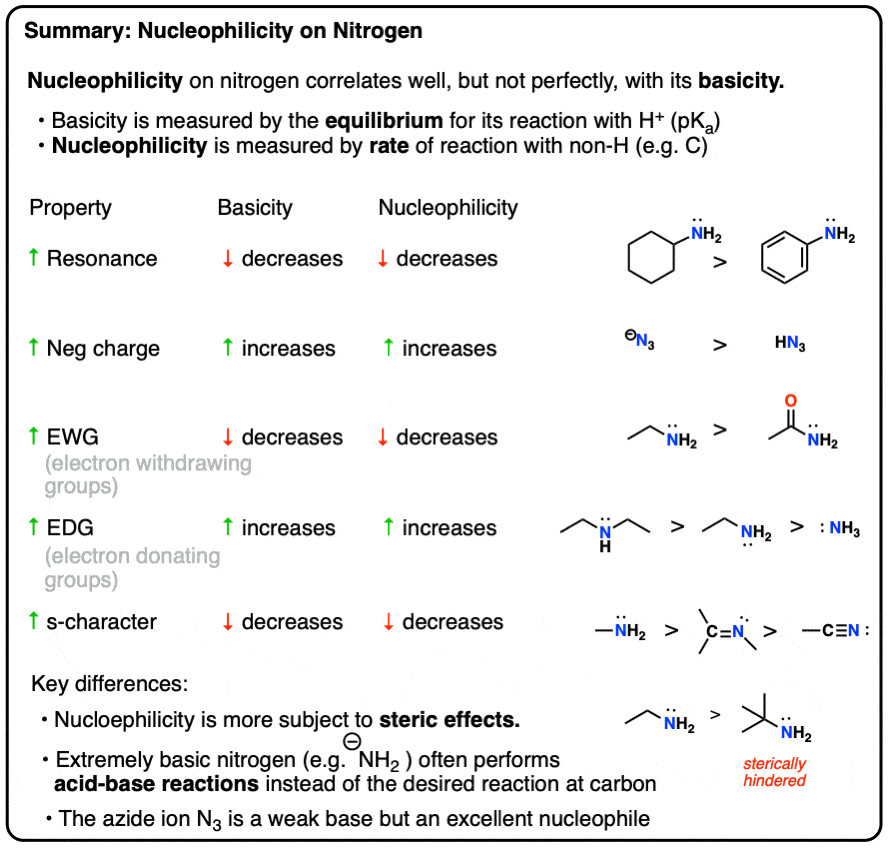
Table of Contents
- A Quick Review: Basicity vs. Nucleophilicity
- How Acidity/Basicity Is Measured vs. How Nucleophilicity Is Measured
- Some Rules Of Thumb For Comparing Basicity and Nucleophilicity
- A Good First Approximation: “The Stronger The Base, The Stronger The Nucleophile”
- Exception #1: “Is This Too Basic For You?” A Cautionary Note About Amide Bases
- Exception #2: Bulky Amines
- Why Is Nucleophilicity Much More Sensitive To Steric Effects Than Basicity?
- Exception #3: Poorly Basic Amines That Are Great Nucleophiles
- Nucleophilicity of Amines: Summary
- Bonus Section: Quantifying The Nucleophilicity Trends of Amines With The Mayr Scale
- Notes
- (Advanced) References and Further Reading
1. A Quick review: Basicity vs. Nucleophilicity
“Um, how are basicity and nucleophilicity different again“? you might ask. Always a good question, because sometimes it takes approaching the subject from a few different angles to really make it sink in. Here, we’ll look at it from the perspective of amines, but a lot of the principles are general.
When Is An Amine Called A “Base” vs. When Is An Amine Called A “Nucleophile”?
- When an amine reacts to form a bond with a proton (H+) we say it is acting as a base. (A Brønsted base, specifically, if you want to tie it back to a familiar concept from general chemistry). The reactive partner of a base is called an “acid”, “Brønsted acid”, or simply a “proton”.
- When an amine reacts to form a bond with any atom other than H, we say it is acting as a nucleophile. The reaction partner is called an electrophile.
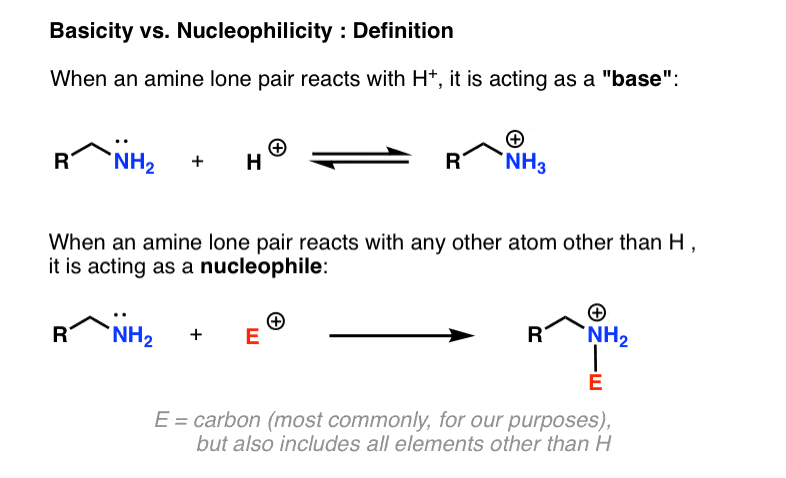
Since the electrophile can potentially be any atom on the periodic table (except for H, which would make nitrogen a “base”), there’s inherently a lot more variability possible for nucleophilicity than there is for basicity. Here, we’re going to confine ourselves largely to the reactions of amine bases with carbon-based electrophiles, since it’s the most relevant for our purposes.
2. How Acidity/Basicity Is Measured vs. How Nucleophilicity Is Measured
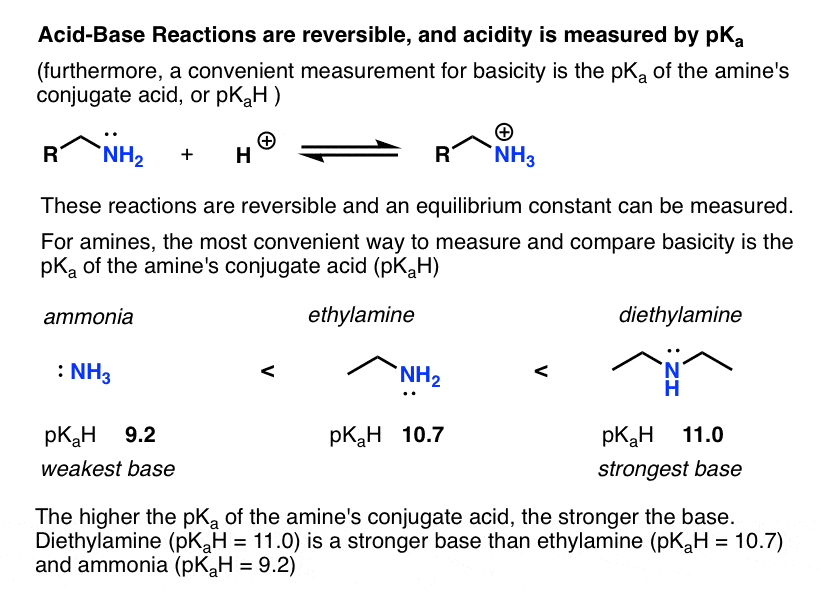
- Acid-base reactions are generally reversible and therefore the equilibrium constant (“acidity constant”) Ka can be measured. The (negative) log of the acidity constant, pKa , measures the strength of acids, and it goes from about –10 for strong acids (–10 for HI ) to over 50 for weak acids (>50 for alkanes).
Since “the weaker the acid, the stronger the conjugate base”, a convenient way to compare the basicity of amines is by the pKa of the conjugate acid (which we can call pKaH). The higher the pKaH, the greater the basicity of the amine. (see: Basicity of Amines and pKaH)
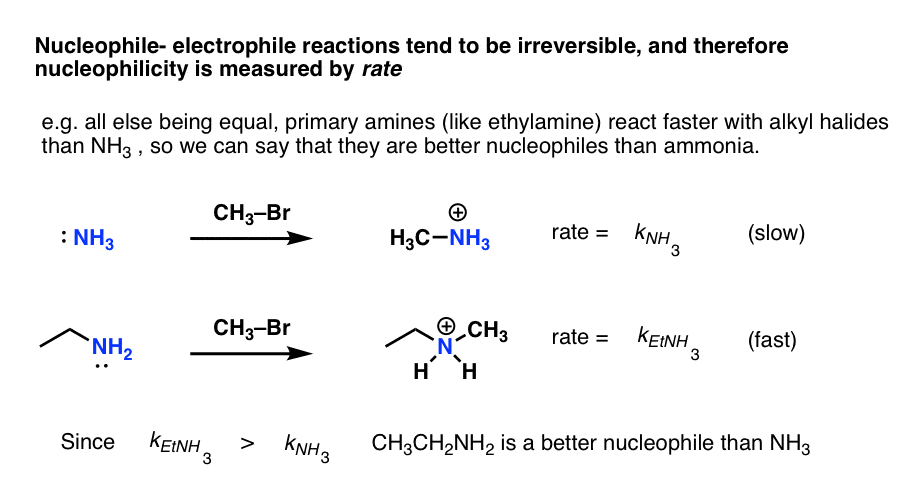
- In contrast, nucleophile-electrophile reactions are generally irreversible and therefore equilibrium constants can’t be readily measured. The only option available is to measure the rate.
So what we call “strong” nucleophile is one that reacts quickly with a given electrophile, whereas a “weak” nucleophile reacts slowly with the same electrophile.
3. Some Rules Of Thumb For Comparing Basicity and Nucleophilicity
The position of an equilibrium (“how stable are two reactants, relative to their products”) and the reaction rate (“how rapidly do two reactants combine to give a product?” ) are two very different measurements. To be sure, there’s a lot of correlation, but it’s just as important to know the situations where the correlations DON’T hold.
To put this in perspective, let’s tie it back to human relationships.
Does the fact that two people fall in love with each other make for a stable long-term marriage?
In fairy tales, the answer is, “of course”. It’s meant to be! What could go wrong?
In real life, the course of love does not always run smooth. That’s why we have tabloids and country songs.
4. A Good First Approximation: “The Stronger The Base, The Stronger The Nucleophile…”
In general, the stronger the base, the stronger the nucleophile (you can think of this as the “fairy tale” scenario I mentioned above).
Think of this sketch, which shows basicity increasing with nucleophilicity (note: this is not drawn to any reasonable scale)
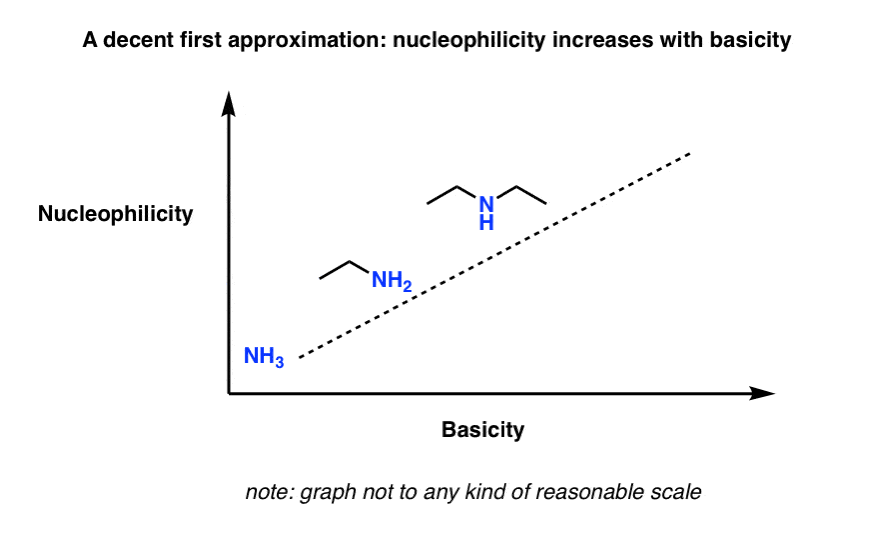
Sometimes helpful to think of “basicity” as: “stability of the lone pair”. Anything which makes the lone pair more stable will decrease the basicity.
[Need a review on the factors which affect basicity? We covered 5 important basicity trends for amines here. Alternatively you can see this footnote, which covers the effect of conjugation, electron donating (and withdrawing) groups, and hybridization.]
So if basicity mostly correlates with nucleophilicity, you might ask: what are the exceptions?
There are three big exceptions to this rule of thumb: 1) strong bases, 2) bulky amines, and 3) a few highly nucleophilic but weakly basic nitrogens.
5. Exception #1: “Is This Too Basic For You?” A Cautionary Note About Amide Bases
The conjugate bases of amines (“amide bases”) such as sodium amide (NaNH2) are extremely strong bases (pKaH about 35–38). If basicity correlates with nucleophilicity, one might expect them to also be great nucleophiles, way up in the right-hand corner of this chart.
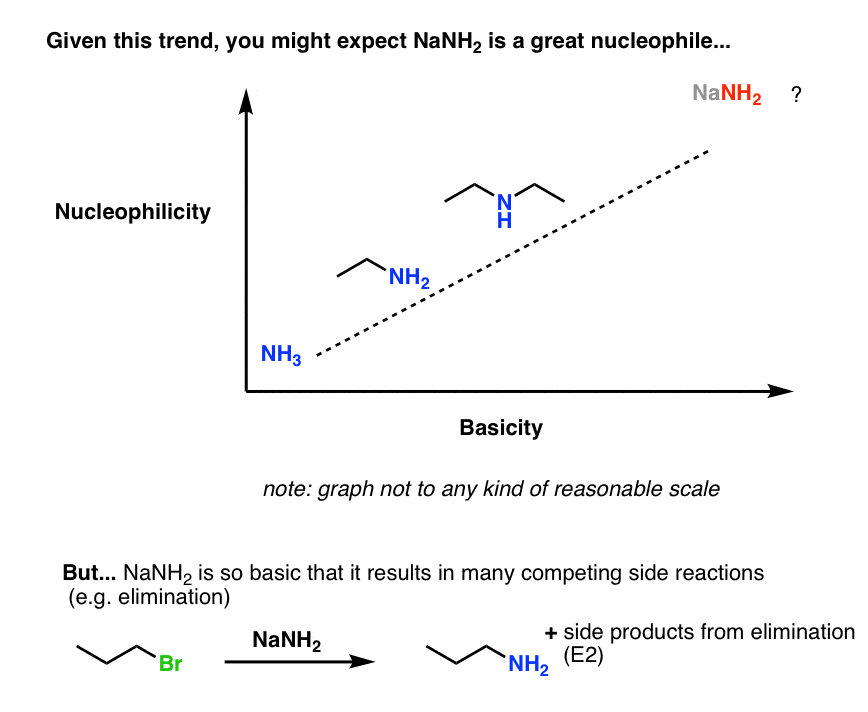
In practice, using an amide base for an SN2 reaction is like trying to do surgery with a skill saw. The extra power doesn’t help very much, and there’s too much potential for collateral damage.
Neutral amines are already decent enough nucleophiles for most purposes (e.g. the SN2) and using an amide base doesn’t confer any great advantage.
The potential drawback in using amide bases as nucleophiles is that acid-base reactions tend to be fast, relative to reactions at carbon [Post: Acid Base Reactions Are Fast], and side reactions can occur.
For example, trying to do an SN2 on an alkyl halide may end up with some of the desired product, but it also has the potential to deliver the (undesired) elimination product as well. Too much basicity can be a detriment.
[This is similar to the reason why Grignard reagents aren’t often used in nucleophilic substitution reactions; they’re extremely strong bases, and other processes can get in the way. ]
Pro tip: a better substitute for using NaNH2 in a substitution reaction is to use highly nucleophilic but relatively non-basic sodium azide (NaN3) followed by reduction of the azide to the amine (e.g. with LiAlH4 ).
6. Exception #2: Bulky Amines.
In acid-base reactions, the electrophile is always the same (H+). But in reactions of nucleophiles, the electrophile can be any number of different atoms on the periodic table, and that can lead to complications.
One way this comes up is that nucleophilicity is much more sensitive to steric effects than is acidity/basicity.
For example, t-butylamine is about as basic as other primary amines but is considerably less nucleophilic.
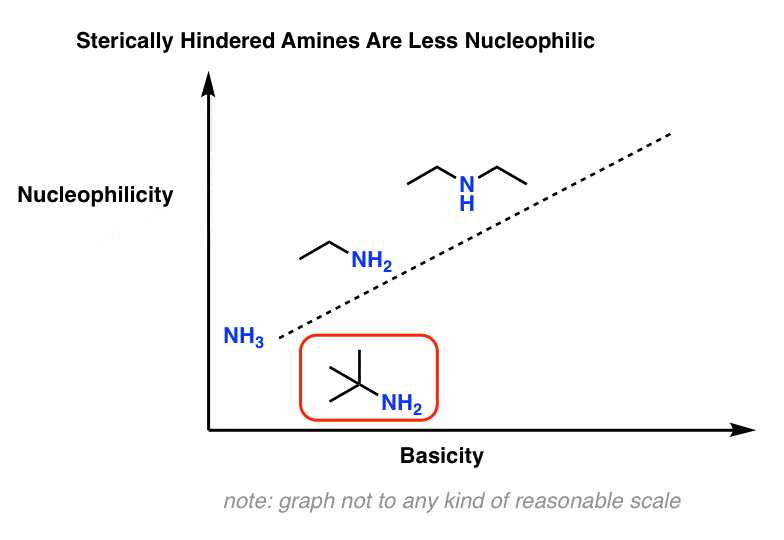
7. Why Is Nucleophilicity Is Much More Sensitive To Steric Effects Than Basicity?
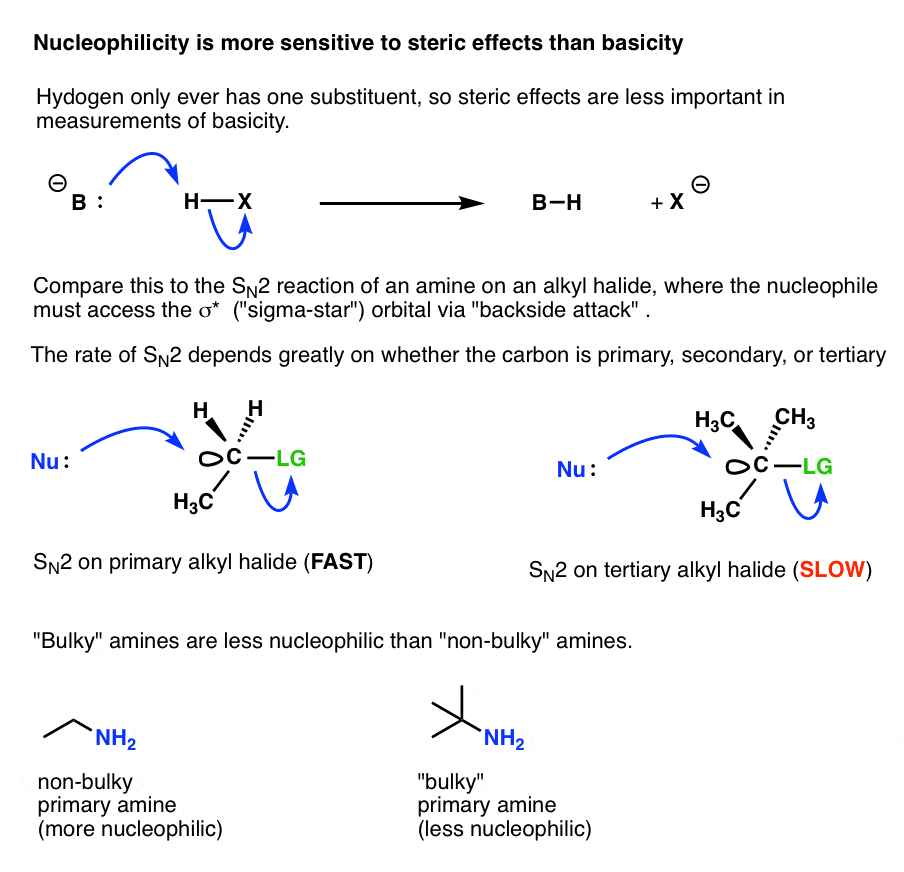
It comes down to the fact that when an amine is acting as a “base”, it is attacking a fundamentally different type of orbital than when it is acting as a “nucleophile” , and those orbitals have very different steric constraints.
- When an amine base reacts with a proton in an acid-base reaction, the amine lone pair just has to come into contact with the spherical 1s orbital of a hydrogen atom, in order for a reaction to occur.
- When an amine nucleophile attacks an alkyl halide, the nucleophile can’t approach the carbon atom from just any direction in order for a reaction to occur. Remember the “backside attack?” The nucleophile has to approach from a very specific direction so that it can contact the sigma* orbital of a carbon-leaving group bond.
Any additional groups attached to that carbon can further constrain the available angles by which the nucleophile can successfully approach the alkyl halide.
This is the source of the familiar rule of thumb that SN2 reactions are fastest with methyl and primary alkyl halides, and slowest with tertiary alkyl halides.
You can also compare it to the great experiment performed by sportswriter Todd Gallagher when the Washington Capitals repeatedly failed to score on a goalie wearing a fat suit. [See: Steric Hindrance Is Like A Fat Goalie]
[Note 2]
8. Exception #3: Poorly Basic Amines That Are Great Nucleophiles
So if there are relatively basic amines which are not good nucleophiles, you might ask: are there relatively non-basic amines which are great nucleophiles?
Yes. See the top-left of this graph:
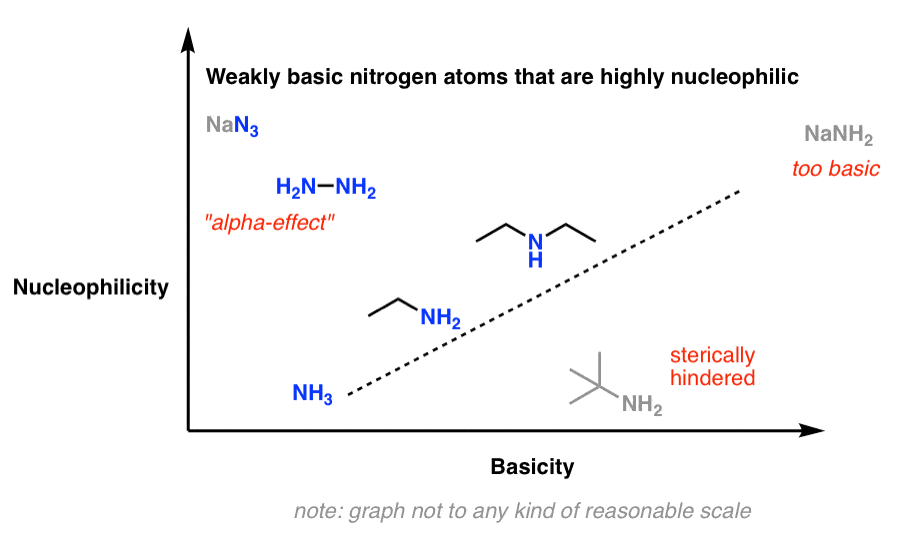
While not technically an amine, the poorly basic (pKaH = 4.7) but highly nucleophilic azide ion (N3 –) immediately comes to mind in this category. Besides being very small and therefore not subject to steric constraints, the azide ion is a “twofer”: each end of the nucleophile can potentially act as a nucleophile. (You can think of this as doubling its effective concentration.)

Two other prominent examples are hydrazine and hydroxylamine, which are both about as basic as ammonia, but are far more nucleophilic. [You may recall that hydrazine is used to cleave phthalimide in the Gabriel synthesis].
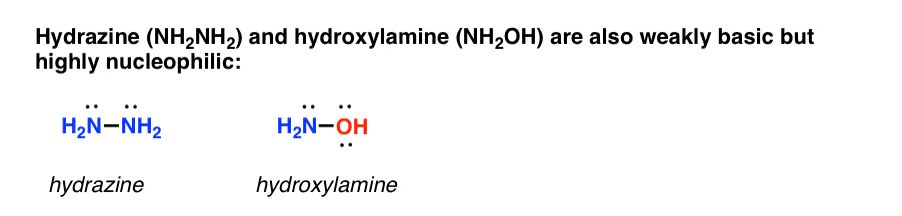
What makes these species particularly nucleophilic?
It’s not perfectly understood, but essentially when a nucleophilic atom is bonded to an atom which also bears a lone pair, the nucleophilicity increases significantly [the energy of the HOMO is increased]. This increase in nucleophilicity is called the “alpha-effect“.
9. Nucleophilicity of Amines: Summary
For amines (and related species, like azides) the general trend is that nucleophilicity increases with basicity, with a few exceptions:
- bulky bases (like t-butylamine) are less nucleophilic than expected, due to steric factors
- the azide ion and amines bearing an adjacent atom bearing a lone pair (e.g. hydrazine, hydroxylamine) are more nucleophilic than expected due to the “alpha-effect”.
- extremely strong bases (such as NaNH2) can lead to acid-base side reactions rather than the desired reaction with the electrophile.
The usual caveats about nucleophiles apply too. For example, polar aprotic solvents are better than polar protic solvents for promoting SN2 reactions, since they don’t hydrogen-bond with the nucleophile (and thus surround it with a bulky shell of solvent).
If you’re OK with a relatively qualitative approach, great!
On the other hand, if you want a slightly more quantitative approach to nucleophilicity, read on.
Bonus Section: Quantifying The Nucleophilicity Trends of Amines
An impressive body of work on the relative power of nucleophiles comes from the lab of Herbert Mayr, who for the past few decades has compiled various reactivity scales for a number of different nucleophiles and electrophiles. Given the large number of variables involved, no scale will ever perfect under all conditions, but the values obtained do give us a relative sense of the impact of various factors in the nucleophilicity of amines.
The Mayr numbers are useful as “ballpark” figures for determining order-of-magnitude electronic and steric effects, so long as the electrophile is not greatly sterically hindered.
Like pKa values, the Mayr nucleophilicity parameters are logarithmic. In the case of the Mayr tables, the higher the number, the better the nucleophile.
We won’t go into the details of how these numbers are determined here, but for the curious there’s plenty of background on the website and in the associated papers. Here is Mayr’s homepage database. Here is the the list of amine nucleophilicities that this part of the post is based on. Here is a handy-dandy presentation discussing Mayr’s work.
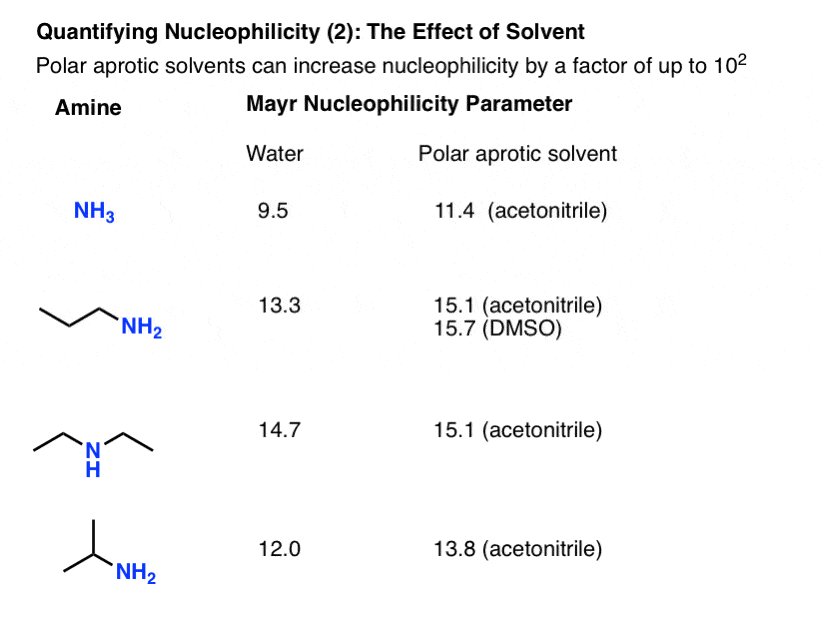
Quantifying The Effect Of Solvent: Using Polar Aprotic Solvents Is Worth It
We’ve tried to stress how polar aprotic solvents increase nucleophilicity over polar protic solvents since the SN2 days in Org 1. Having a set of nucleophilicity parameters allows us to answer: how much?
The answer appears to be by at least a factor of 100, if we compare the nucleophilicity parameters of ammonia in water and acetonitrile. All else being equal, that’s the difference between waiting 15 minutes for your reaction to complete versus waiting a full day, just by choosing a different solvent. If time is money, what does that make polar aprotic solvents?

The Main Trend: Correlation of Basicity With Nucleophilicity
We mentioned that the nucleophilicity of amines proceeds in the order NH3 < primary amines < secondary amines, and correlated this with pKaH. The Mayr nucleophilicity parameters have the same broad trend.
For water as solvent, the nucleophilicity parameter for NH3 is 9.5, for ethylamine 12.9 (about 1000 times more nucleophilic) and for diethylamine 14.7 (about 100 times more nucleophilic than ethylamine and 100,000 times more nucleophilic than ammonia.)
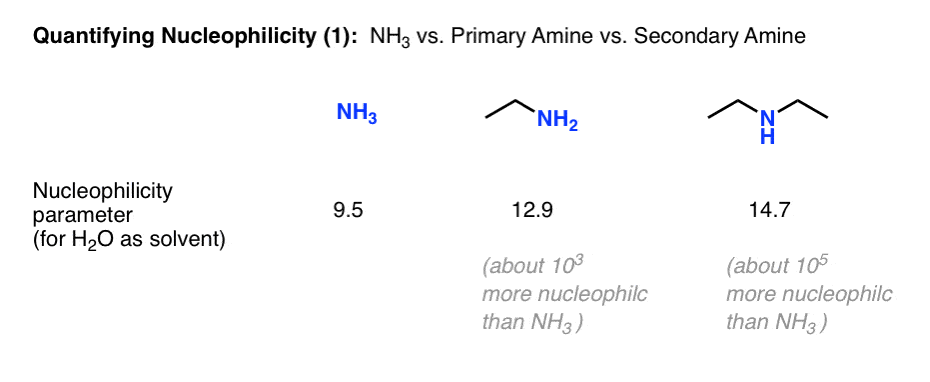
This starts to put some perspective on the question of why the reaction of ammonia with alkyl halides is not a useful reaction for preparing primary amines; the product is 1000 times more nucleophilic!
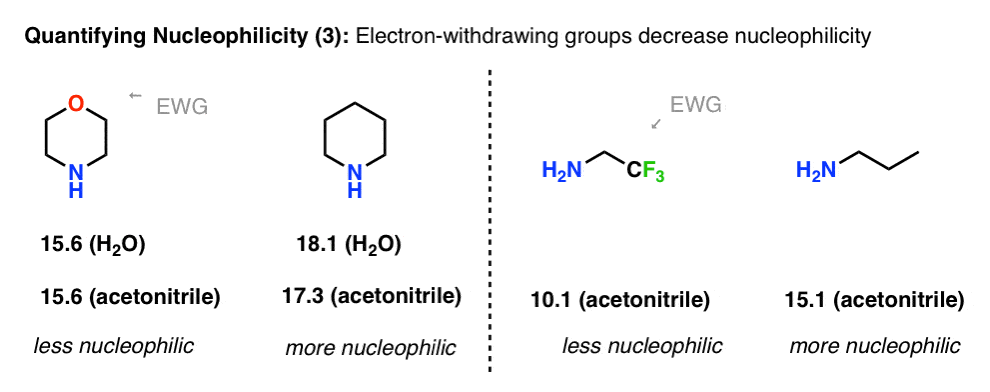
Another example where basicity correlates with nucleophilicity is in the effect of electron-withdrawing groups. Compare the nucleophilicity of piperidine (18.1 in H2O) with morpholine (15.6 in H2O). The electron-withdrawing oxygen has the effect of reducing nucleophilicity by a factor of 300.
With 2,2,2-trifluoroethylamine, the trifluoro group reduces nucleophilicity by about 100,000.
Quantifying The Effect Of Steric Hindrance
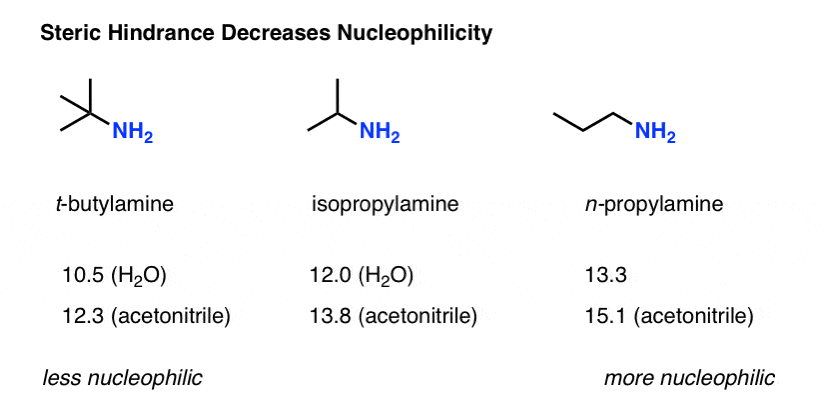
We mentioned that steric hindrance reduces the nucleophilicity of amines. By how much?
The Mayr parameters for t-butylamine, isopropylamine and n-propylamine show a clear trend, going from (in water as solvent) 10.5 (in water) for t-butylamine up to 12.0 for isopropylamine to 13.3 for n-propylamine.
So as a rough gauge, the t-butyl group reduces the nucleophilicity by a factor of about 1000 versus a “normal” primary amine.
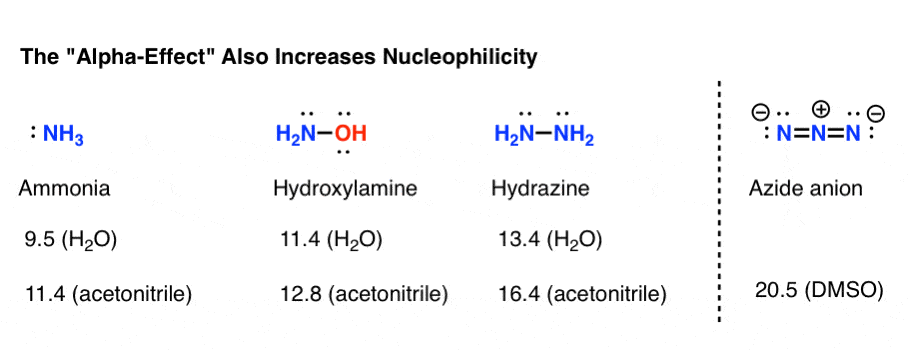
Highly nucleophilic amines that aren’t very basic
The measured set of nucleophilicity parameters give an estimate that hydroxylamine is about 100 times more nucleophilic than ammonia (in water), while hydrazine is about 10,000 times more nucleophilic.
The nucleophilicity parameter for the azide ion wasn’t measured in water, but if we can compare two different polar aprotic solvents (DMSO and acetonitrile) it’s about a billion times more nucleophilic than NH3.
Useful tidbit: never, ever use CH2Cl2 or chloroform as solvent for any reaction involving the azide ion. The azide ion is so nucleophilic it will displace the chlorides, leading to the formation of a potentially explosive diazidomethane.
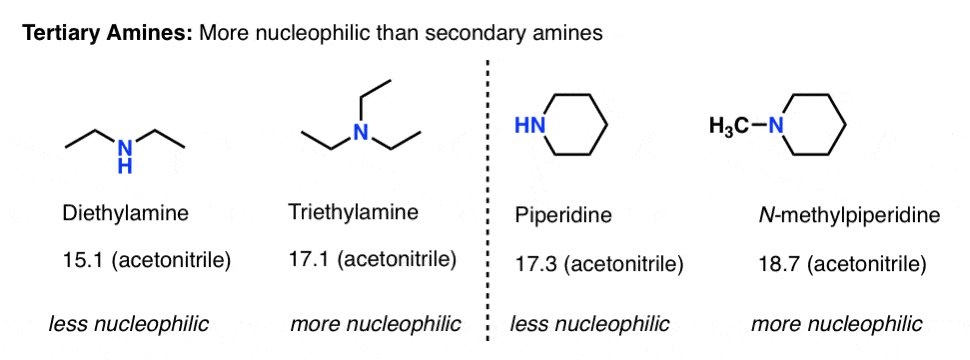
What About Tertiary Amines?
Conspicuously absent from this whole discussion has been the question of tertiary amines.
- On one hand, tertiary amines have an extra alkyl group attached to nitrogen, so we’d expect them to be more basic (and nucleophilic) than secondary amines. [In fact, triethylamine is slightly less basic than diethylamine in water, largely because of solubility factors].
- On the other hand, tertiary amines should be more sterically hindered than secondary amines, reducing nucleophilicity.
So are tertiary amines better or worse nucleophiles than secondary amines? Which factor “wins” ?
The Mayr nucleophilicity factors can help sort out this question.
It’s hard to see trends with water as a solvent because most tertiary amines are not very soluble in water.
In acetonitrile, it looks like tertiary amines are at least one, if not two orders of magnitude more nucleophilic than comparable secondary amines.
A particularly interesting example of a highly nucleophilic tertiary amine is quinuclidine, where the three alkyl groups are “tied back” in a bicyclic structure. The nucleophilicity paramter of quninuclidine in acetonitrile is 20.5, on par with the azide ion.
A caveat: Remember that the Mayr parameters work best with non sterically hindered electrophiles. So expect the reaction rate of a tertiary amine with a hindered electrophile to fall off a cliff, relative to a less hindered electrophile.
Notes
- Basicity (and nucleophilicity) is reduced by conjugation, when a lone pair can be delocalized through resonance (e.g. aminobenzene is less basic than cyclohexylamine, and less nucleophilic also.)
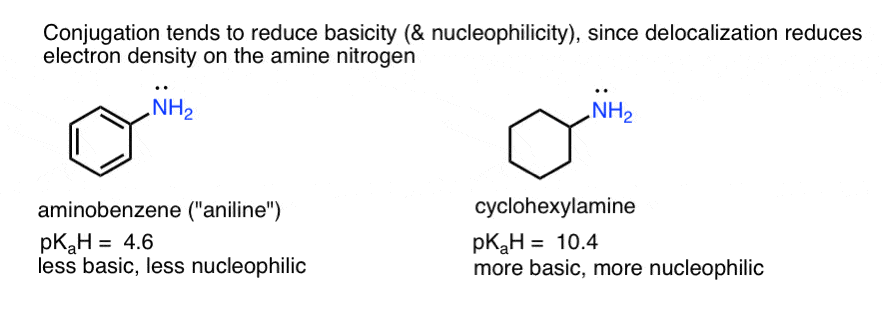
- Electron withdrawing groups reduce basicity (and nucleophilicity), such as in morpholine versus piperidine (sigma acceptors) and acetamide (pi acceptor) versus ethylamine.
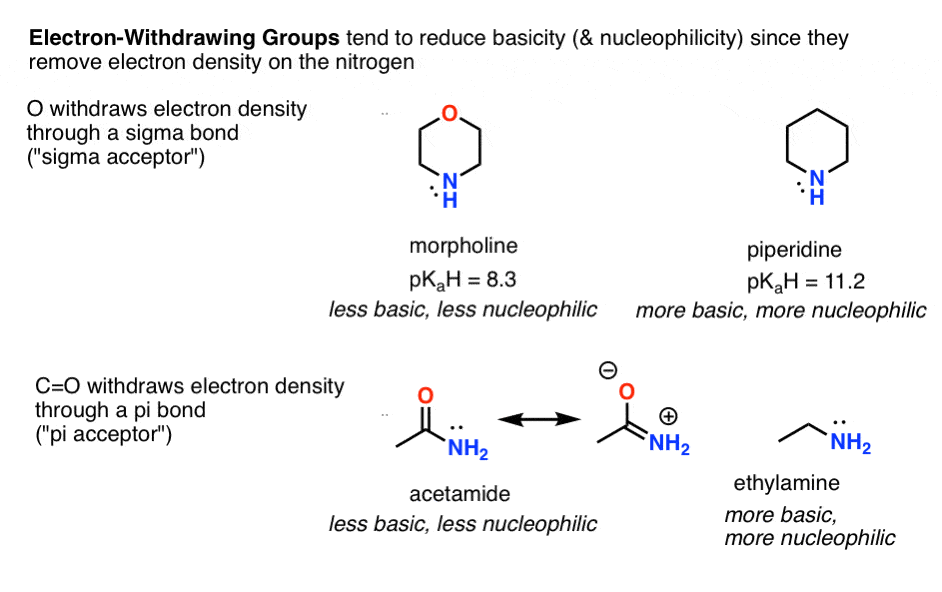
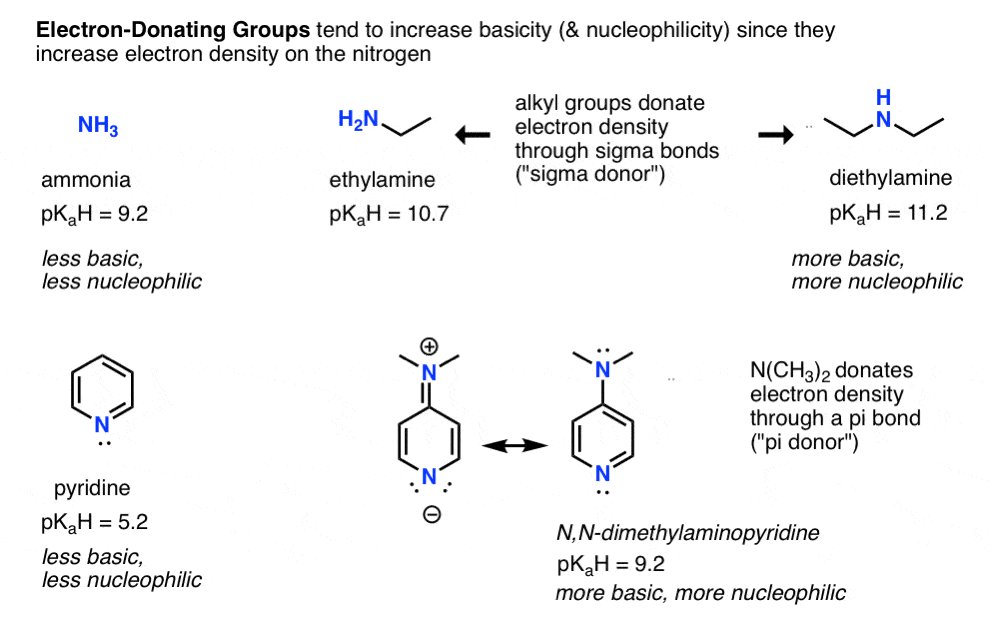
- Conversely, electron donating groups increase basicity (and nucleophilicity) such as in alkylamines and 4-dimethylaminopyridine vs. pyridine (pi donors).
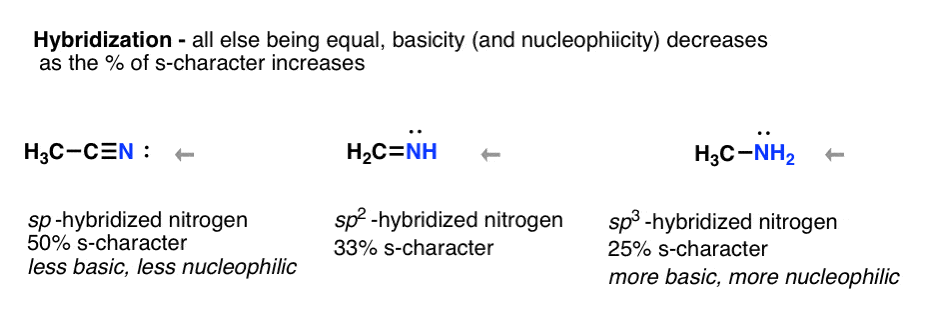
- Increasing the s-character of the orbital decreases basicity (and nucleophilicity) sp < sp2 < sp3
Note 2. In this post we’re mainly discussing nitrogen nucleophiles and carbon electrophiles, but when dealing more broadly on the subject of nucleophilicity the issue of orbital overlap between orbitals on different rows of the periodic table can arise. Sometime in the future we can address hard soft acid base (HSAB) theory. On the other hand, at a lecture I saw Mayr give once on his reactivity tables, he was asked where hard and soft acid-base theory effects were in his data. His answer was, “I don’t know”, by which he meant, he thought his data doesn’t seem to support it.
(Advanced) References and Further Reading
- The Nucleophilicity of Amines
William A. Henderson and Carol J. Schultz
The Journal of Organic Chemistry 1962, 27 (12), 4643-4646
DOI: 10.1021/jo01059a507
Interestingly, this paper studies the nucleophilicity of various amines and shows that reactivity goes in the order primary > secondary > tertiary.Herbert Mayr (LMU, Munich, Germany) has spent his career in fundamental Physical Organic Chemistry creating scales for the quantification of electrophilicity and nucleophilicity. He has several papers where he has carefully compared the rates of reaction of various nucleophiles against a reference electrophile (usually benzhydrylium cation), or various electrophiles against a reference nucleophile. This allows the prediction of reaction rates from empirically obtained parameters. - Nucleophilicities of Primary and Secondary Amines in Water
Frank Brotzel, Ying Cheung Chu, and Herbert Mayr
The Journal of Organic Chemistry 2007, 72 (10), 3679-3688
DOI: 10.1021/jo062586z
This paper gives experimental kinetic evidence for the general rule that secondary amines are more nucleophilic than primary amines. - Nucleophilicities of Amines, Amino Acids, and Pyridines.
Frank Botzel’s PhD Thesis, under the guidance of Prof. Herbert Mayr, titled “Nucleophilicities of Amines, Amino Acids and Pyridines”. PhD theses are often a useful resource for unpublished results or other data that may have not made it to a publication. - Nucleophilic Reactivities of Primary and Secondary Amines in Acetonitrile
Tanja Kanzian, Tobias A. Nigst, Andreas Maier, Stefan Pichl, Herbert Mayr
J. Org. Chem. 2009, 36, 6379-6385
DOI: 10.1002/ejoc.200900925
This paper also demonstrates that on average, secondary amines are stronger nucleophiles than primary amines – as Fig. 4 shows, there are some primary amines that are stronger nucleophile than some secondary amines. - Nucleophilic Reactivities of Hydrazines and Amines: The Futile Search for the α-Effect in Hydrazine Reactivities
Tobias A. Nigst, Anna Antipova, and Herbert Mayr
The Journal of Organic Chemistry 2012, 77 (18), 8142-8155
DOI: 10.1021/jo301497g
The a-affect describes the enhanced reactivity of nucleophiles which bear an unshared pair of electrons at the atom adjacent to the nucleophilic center. However, this could not be observed for amines – methylamine is more nucleophilic than hydrazine. - Nucleophilicity parameters for amines, amino acids and peptides in water. Variations in selectivities for quinone methides
William Bentley
Org. Biomol. Chem., 2011, 9, 6685-6690
DOI: 10.1039/C1OB05715D
Hello,
I am trying to get Si-Cl and -NH-Si- to react together in order to form Si-N-Si + HCl. I would like to know if the NH is nucleophilic enough to have the reaction between the electrophilic Si-Cl and the amine. this reaction works with Si-OH and Si-Cl so I would like to transpose it to amine counterpart.
Yes, it will definitely work, for example look up the synthesis of hexamethyldisilazane. https://en.wikipedia.org/wiki/Bis(trimethylsilyl)amine
Hi James, thank you for a really interesting topic !
I’m currently working with nitriles, and I’ve been unable to find a good quantification method of their nuclophilicity, weither looking into Mayr’s of Hammet’s quantifying methods. Do you know if there’s any ?
Thank you in advance !
Guillaume
Hi Guillaume – nitriles or cyanide ion? Nitriles themselves are really bad nucleophiles and will only work in Ritter-type reactions where they are reacting with an extremely good electrophile.
Think about hybridization. Acetylene C-H is more acidic than alkanes because sp-hybridization stabilizes negative charge. Likewise the nitrogen of nitriles, being sp-hybridized makes that nitrogen lone pair very stable (and a very poor base / nucleophile) relative to the sp3 hybridized nitrogen in amines.
I am stuck with carrying out a nucleophilic substitution reaction of triethylamine with chloride atoms in Polyvinyl chloride. Could you gove some ideas please?
How do you know it’s going to do a substitution and not elimination?
Hi James,
I routinely conjugate proteins via their primary amines to substrates and tags containing NHS esters at pH 7.2. It just occurred to me that at pH 7.2 the primary amine should be protonated to R-NH3(+), since the pKa of primary amines in proteins is approximately 9 and 10.5, for alpha and epsilon amines, respectively. How can the positively charged, protonated amine act as a nucleophile?? Thanks in advance, and I love reading your page!
Rich
The pKa values you cite are for the conjugate acids of the amines, not the amines themselves. pH 7.2 is a neutral solution. The neutral amines will not be protonated to any appreciable extent until pH4 or below, and even then they will be in equilibrium.
Very good explanation but could you clarify the influence of H2O in section about quantifying nucleophilicity? Water is polar protic solvent so why should the nucleophilicity of the amines (NH3 vs secondary vs tertiary) increase in water? Shouldn’t it be the other way around due to H-bonding?
Right. The nucleophilicity will certainly be solvent dependent. If you look at the tables from Mayr they all specify solvent and only should be compared directly when the numbers were obtained in the same solvent. You will also note that tertiary amines don’t have good nucleophilicity numbers in water, because they’re just not very soluble in polar solvents.
I don’t have a good clear answer for you regarding trends. Hydrogen bonding is one factor, but so is the increased electron density that comes with additional alkyl groups. It’s not a one-dimensional quantity, it’s multivariable. Making a measurement is the best way to sort it all out.
What can be done to increase the nucleophilicity of amines? what kind of base can be used for this purpose?
One way would be to deprotonate a primary or secondary amine to yield its conjugate base, but you’d likely run into problems with competing acid-base reactions.
Very helpful posts!
How to compare the nucleophilic ability of phenol,thiophenol and aniline?
Hello I have the task where tertiary anime reacts with 3-clorpentane. So if I am correct it is an SN2 reaction ant the product will be amonium salt. But I am not sure why the reaction rate would increase if we replace solvents in this order: Tertiary-buthanol, ethanol, methanol and water? If you could help me I would be very grateful :)
Think about the influence of solubility on reaction rate and how each of those solvents will affect the solubility of a tertiary amine.
I have a question about the using N-methyl hydrazine as a nucleophile. Both nitrogens experience the alpha effect from each other, right? The primary “amine” nitrogen is less sterically hindered but the secondary “amine” nitrogen experiences the sigma donating effect of the methyl group. Which of the two nitrogens is more nucleophilic then? Thanks!
I haven’t looked at the nucleophilicity table for mono-methyl hydrazine but I would expect that the methylated nitrogen will be more nucleophilic. Mayr has looked a bit at “alpha effects” and it seems to be complicated. https://pubs.acs.org/doi/abs/10.1021/jo301497g?journalCode=joceah
Why does -NH2 shows more mesomeric effect than that of -NHR and -NR2 (where R denotes alkyl group)?
This is actually a great question.
In order for the lone pair to donate into the aromatic ring, it has to adopt a conformation where the substituents on nitrogen are in the same plane as the aromatic ring. As steric bulk increases on the nitrogen, this conformation will become less favorable due to allylic strain.
So it’s not so much that NH2 is more nucleophilic, but that the conformations of NHR and NR2 are less favorable.
Hi James,
First, I think the blog is fantastic. I’ve been reading it for years and your passion comes off the page. Sometimes there is a turn of phrase or a description that helps when thinking about something differently, making it all the clearer.
Second, here are a couple of papers from Mayr relating to, specifically, the alpha effect and HSAB for interest.
https://pubs.acs.org/doi/10.1021/jo301497g
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201007100
Cheers
Thank you very much for this – I love Mayr’s work.
how strong is the nitrogen in acetonitrile? I’ve always had that question. why is it so much weaker compared to the oxygen in carbonyl groups? Thanks!
Very poor nucleophile. Hard to compare with sp2 hybridized oxygen, but likely similar.
Clear basicity trend for nitrogen is:
sp < sp2 < sp3 nitrogen i.e. nitrogen of nitrile < nitrogen of imine < nitrogen of amine Why? Less s-character, lone pair held more closely to nucleus, more stable. More stable = less basic (recall how terminal alkynes are easier to deprotonate than alkanes. Same phenomenon going on here!)
The triple bond also causes a reasonable amount of electrostatic repulsion from the neighboring to electrophile groups. For the same reason acetylenides will only do SN2 with primary alkyl halides but won’t substitute the secondary (will react as a base instead).