Alkene Reactions
Alkene Addition Reactions: “Regioselectivity” and “Stereoselectivity” (Syn/Anti)
Last updated: March 20th, 2024 |
Alkene Addition Reactions: Regioselectivity and Stereoselectivity
- Almost all reactions of alkenes we will learn about can be classified as addition reactions
- In an alkene addition reaction, a C-C pi bond breaks, and two new single bonds to carbon are formed
- Depending on the structure of the alkene and the bonds formed/broken in the reaction, a mixture of constitutional isomers may be formed. In alkene addition reactions, these are often called “regioisomers”.
- When formation of one regioisomer is favored this is called “regioselectivity“. The most common example of regioselectivity is “Markovnikov” selectivity observed in addition of H-X to alkenes, where the C-H bond forms on the “least substituted” carbon (fewest carbons directly attached) and the C-X bond forms on the “most substituted” carbon (most carbons directly attached). The opposite of “Markovnikov” regioselectivity is “anti-Markovnikov” regioselectivity, which is observed in two cases (hydroboration and free-radical addition of HBr).
- The stereoselectivity of alkene addition reactions is also very important! The pi bond of an alkene is flat, and therefore has two faces where addition can occur. When the two new bonds to carbon are formed on the same face, this is called, “syn” addition. When the two new bonds to carbon are formed on opposite faces, this is called, “anti” addition.
- The stereochemistry of the addition reaction is highly dependent on the mechanism of the reaction. Some reactions are selective for syn addition, some reactions are selective for anti addition, and some reactions provide a mixture of syn and anti addition (“unselective”).
- When learning a new alkene addition reaction, there are three key pieces of information you will need to successfully draw the products: 1) the bonds that form/break, 2) the regioselectivity, and 3) the stereoselectivity
- When drawing out the products of an alkene addition reaction, don’t forget to draw out the addition products from both the “top” and “bottom” faces. Depending on the structure of the alkene, and the nature of the reaction, this can lead to formation of constitutional isomers, enantiomers, diastereomers, or even identical products.
- Determining how the products of an alkene addition reaction are related is a very common type of exam problem!
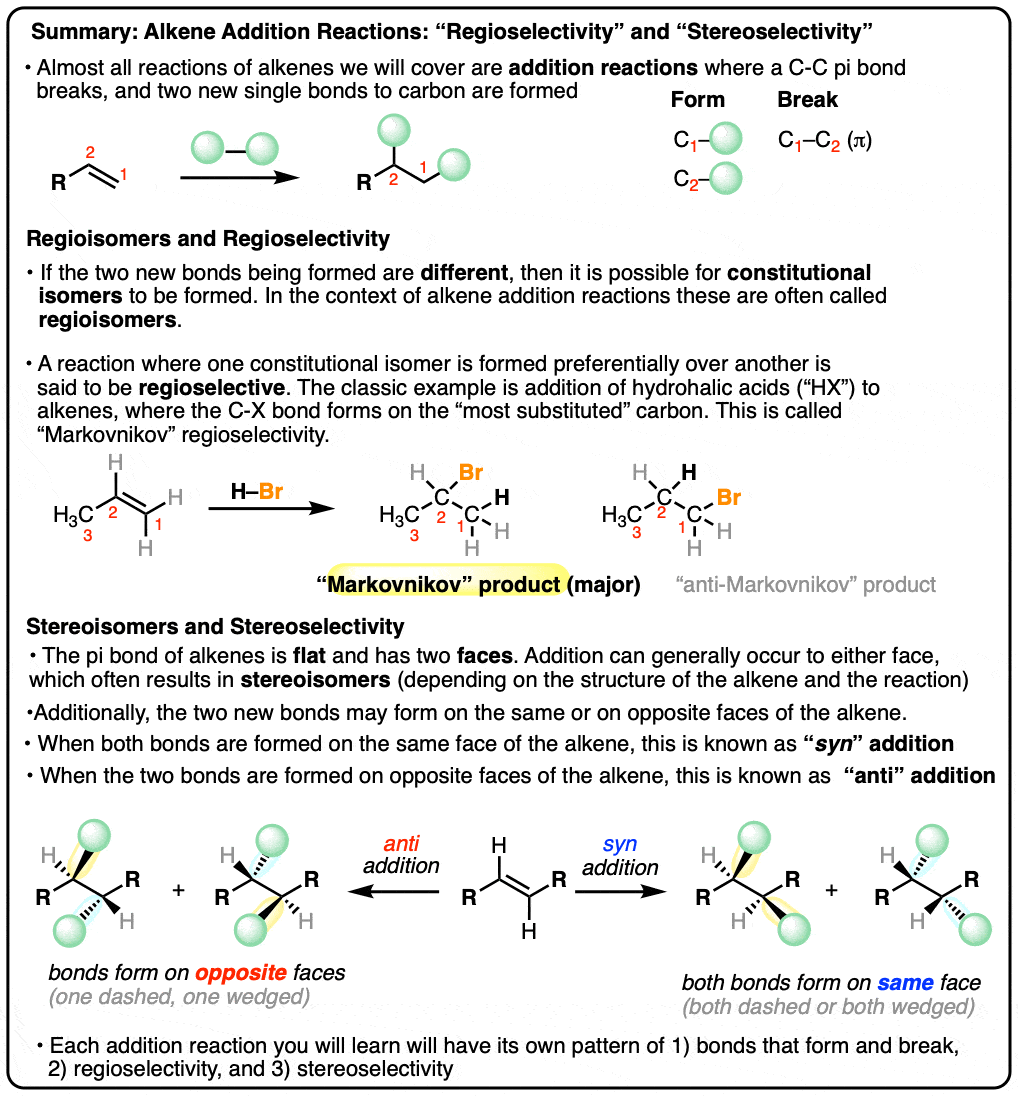
Table of Contents
-
- Alkene Reactions Follow A Few Key Patterns
- Alkene Addition Reactions
- Regioisomers in Alkene Addition Reactions: Markovnikov vs. anti-Markovnikov Regioselectivity
- Syn and Anti Addition To Alkenes
- Stereoselectivity in Alkene Addition Reactions
- Draw Out The Product Of Addition To Each Face Of The Alkene, Then Determine Relationships
- Summary
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Alkene Reactions Follow A Few Key Patterns
In the articles that follow in this chapter, we are going to cover the main reactions of alkenes. In this article, I want to try to help you recognize the key pattern of bonds that form and break (“addition”) and some of the terms we use to describe the sub-patterns of “regioselectivity” (Markovnikov vs. anti-Markovnikov) and “stereoselectivity” (syn vs anti).
If you take a sneak peek at the Reaction Map of alkenes at the end of this chapter, you would be forgiven for thinking there are an intimidating number of alkene reactions to learn – over 20, depending on how you count. [See article – Reactions of Alkenes]
However, it’s actually a lot easier than it seems, because these reactions follow predictable patterns. And there aren’t 20 patterns – there’s more like three major patterns (with two very minor patterns).
In each of these reactions the specific identity of the bonds that form on the two carbons of the alkene may change, but they will still obey the key patterns.
2. Alkene Addition Reactions
Almost all reactions of alkenes we will learn can be classified as “addition” reactions [Note 1 ]
In an addition reaction, the C-C pi bond is broken, and two new single bonds to carbon are formed.
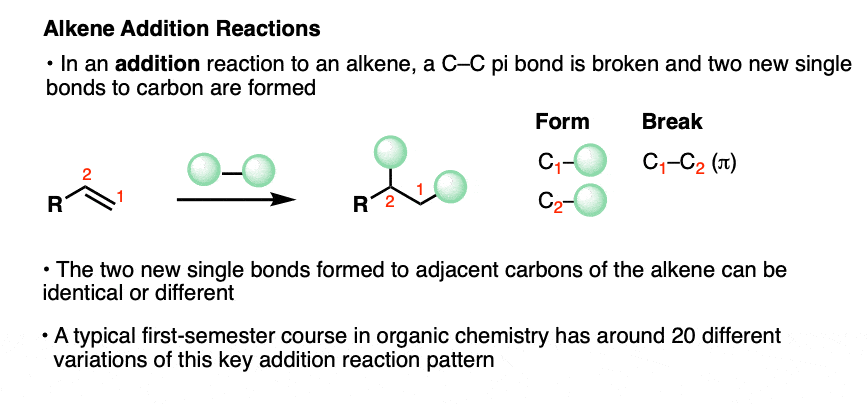
The reactions below are specific examples of alkene addition reactions. At this point you don’t need to know how they work or anything else about them. We’ll cover them in detail in subsequent articles!
Just observe the pattern of bonds that form and break. We break a C-C pi bond (about 60 kcal/mol) and form two new single bonds to carbon.
If you’ve previously covered elimination reactions, the pattern above might look somewhat similar. In an elimination reaction, a new pi bond is formed, and two single bonds to carbon are broken.
In other words, addition is the exact opposite of elimination. hover for image or click this link.
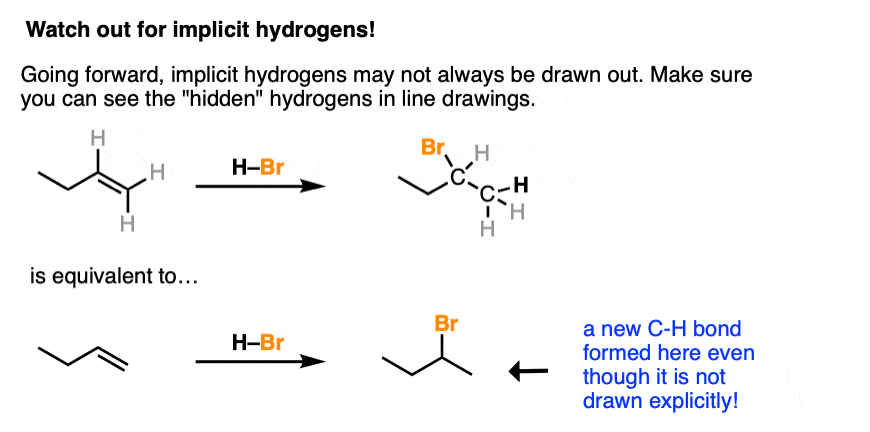
Going forward, make sure you can see the “hidden” (or “implicit”) hydrogens because they will often not be drawn out. You’ll be expected to be able to “see” that a new C-H bond formed here even if it isn’t drawn out explicitly.
3. Regioisomers in Alkene Addition Reactions, and The Three Patterns Of Regioselectivity
Some addition reactions involve forming two identical single bonds to carbon, and others involve forming two different bonds to carbon.
If the two new single bonds to carbon are identical, then an addition reaction will form only one constitutional isomer.
In hydrogenation, for example, two new C-H bonds are formed and a C-C pi bond is broken. There is only one way of ordering the new C-H bonds, so only one constitutional isomer (regioisomer) can form. There can certainly be a mixture of stereoisomers (see below) which have the same connectivity, but have different arrangement of atoms in space. More on that in a bit.
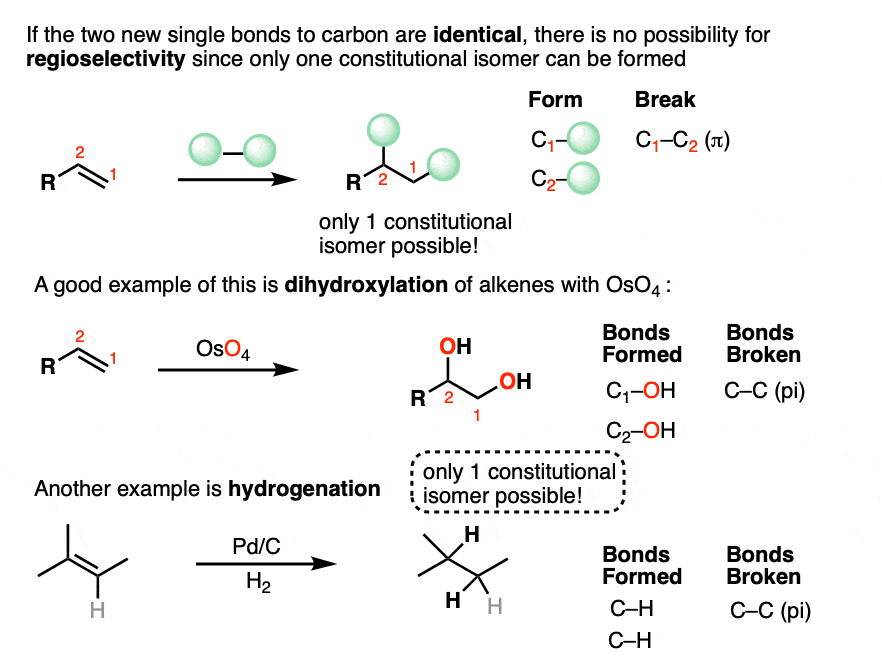
If the alkene addition reaction forms two different bonds to carbon, then there are two different ways of ordering them.
This will only result in formation of a single constitutional isomer if the alkene is completely symmetrical, such as cyclohexene.
hover to see it, or click this link.
If the alkene is not symmetrical – the vast majority of cases! – a mixture of constitutional isomers are formed. We’ll discuss the possibility for stereoisomers below.
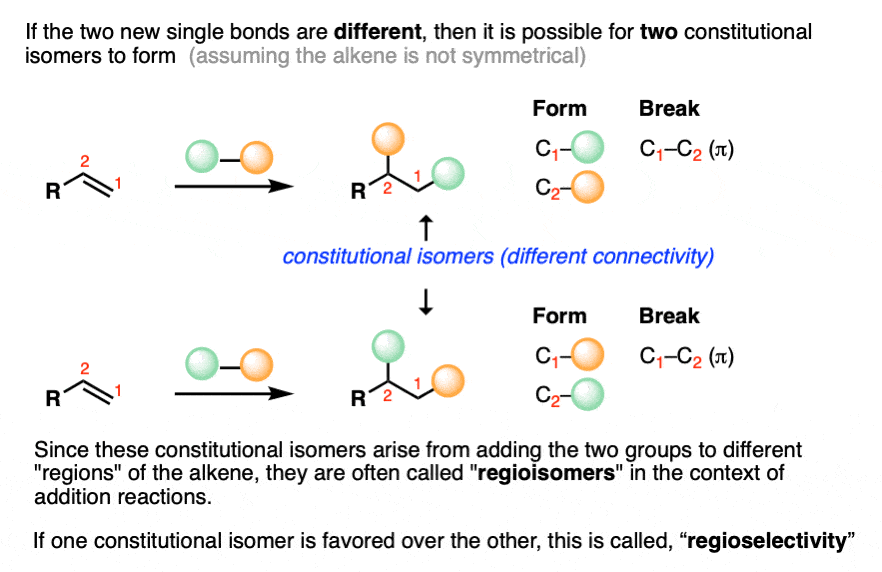
If this pattern were random, then we’d get a 1:1 mixture of products (constitutional isomers) – in other words, a reaction completely lacking in regioselectivity.
As it turns out, the reactions we are going to learn all have predictable patterns of regioselectivity and knowing the pattern will allow us to predict in advance which constitutional isomer will be formed as the major product.
The classic regioselectivity pattern is known as “Markovnikov” regioselectivity and was first observed back in 1870 by Victor Markovnikov in the addition of H–X to alkenes, where a new C-H and C-X bond is formed. [See Hydrohalogenation of Alkenes and Markovnikov’s Rule] (note that X here is a halogen, such as Cl, Br or I).
These reactions tend to happen in a way such that the C–X bond forms to the alkene carbon bearing the most carbon substituents (the “most substituted” carbon) and the C-H bond forms to the carbon bearing the most hydrogens (“less substituted” carbon).
We’ll commonly use the term “Markovnikov-selective” to refer to this pattern of regioselectivity.
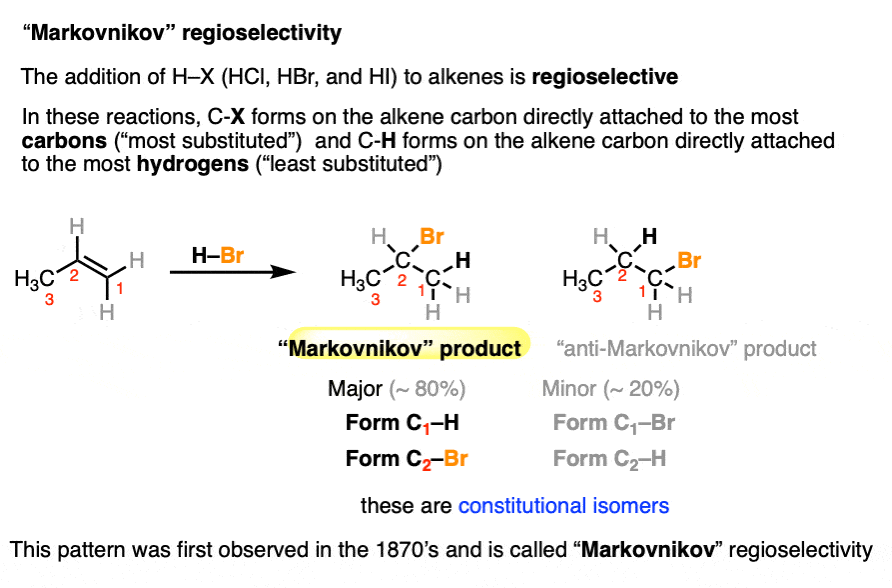
Markovnikov in 1870 had no idea why these reactions had the selectivity that they did.
We now know that the reaction proceeds through a carbocation intermediate. Since carbocations are unstable reaction intermediates with less than a full octet of valence electrons, the reaction will favor the reaction pathway that passes through the most stable carbocation intermediate, which is generally the carbon which is bonded to the greater number of carbon substituents. [See article: Carbocation Stability] . This results in the observed product!
To see the mechanism, hover here or click this link.
Looking ahead a bit, we can apply the same rationale to reactions that pass through positively charged 3-membered ring intermediates such as bromonium ions. (See article – Halogenation of Alkenes). Attack of the nucleophile on these intermediates always occurs to the carbon best able to support positive charge, which can be thought of as another expression of “Markovnikov” selectivity. hover to see or click this link.
If both sides of the alkene are attached to the same number of carbons, then the carbocation intermediates will be of roughly equal stability, and Markovnikov regioselectivity will not manifest itself.
A few reactions (hydroboration and free-radical addition) show the opposite pattern of regioselectivity, where the C-H bond ends up being bonded to the less substituted carbon and C-X to the more substituted carbon.
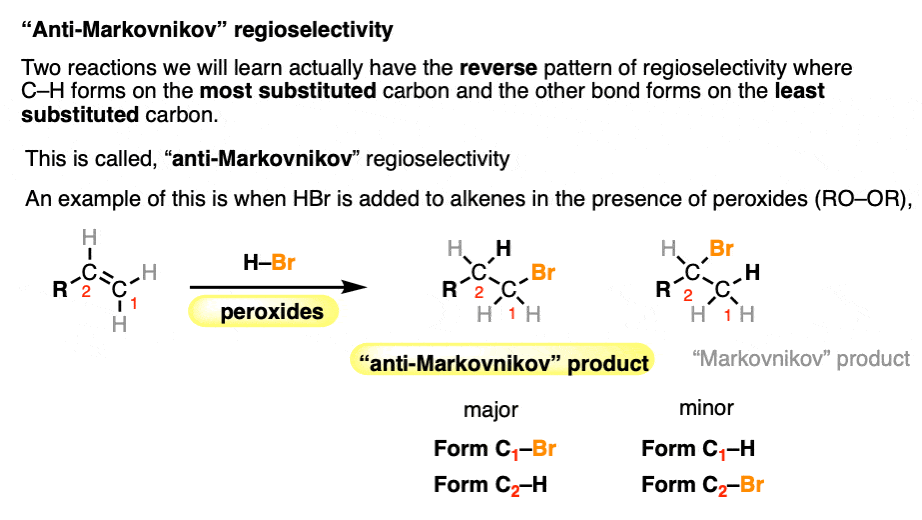
This is somewhat unimaginatively called “anti-Markovnikov” regioselectivity. For more details, see Hydroboration-Oxidation, and Free-Radical Addition of HBr To Alkenes.
To sum up, every single one of the 20+ addition reactions you will learn can be grouped into one of these three patterns of regioselectivity:
- None, or not applicable (N/A)
- Markovnikov selective
- anti-Markovnikov selective
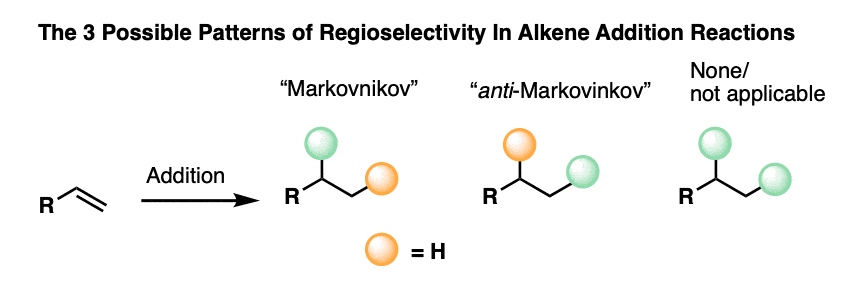
4. Syn and Anti Addition To Alkenes
The pi bond of an alkene is flat (planar). The two sides of an alkene are called its faces.
When an alkene undergoes an addition reaction, the two new single bonds can either form on the same face of the alkene, or they can form on opposite faces.
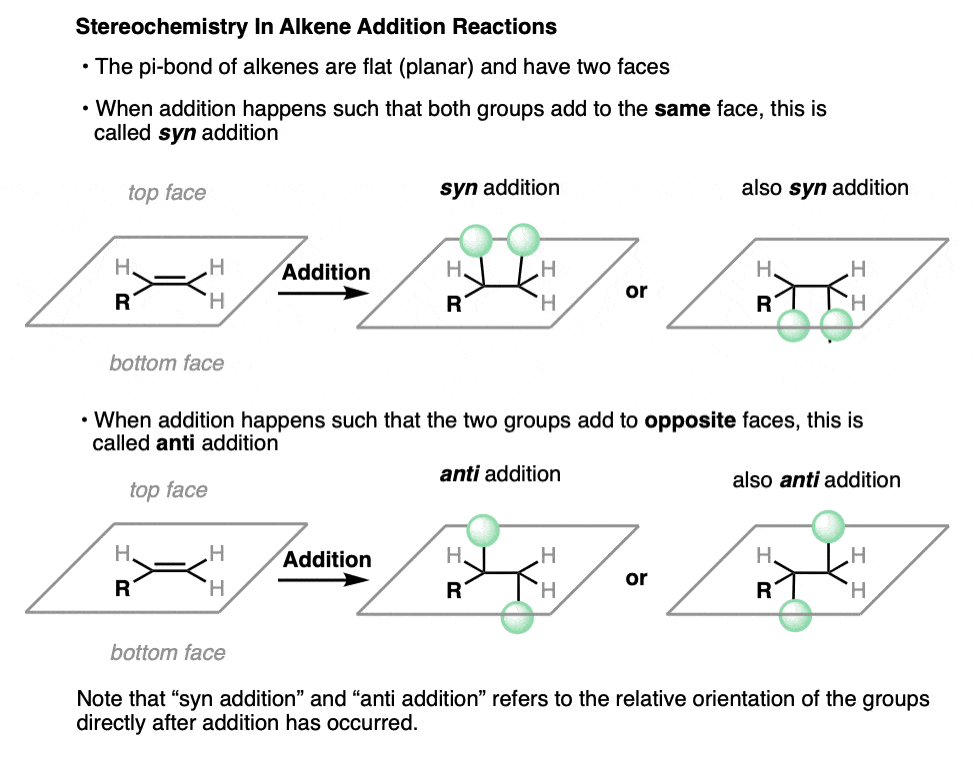
When the two bonds form on the same face of the alkene, we call this syn addition. When they form to opposite faces of the alkene, we call this anti addition.
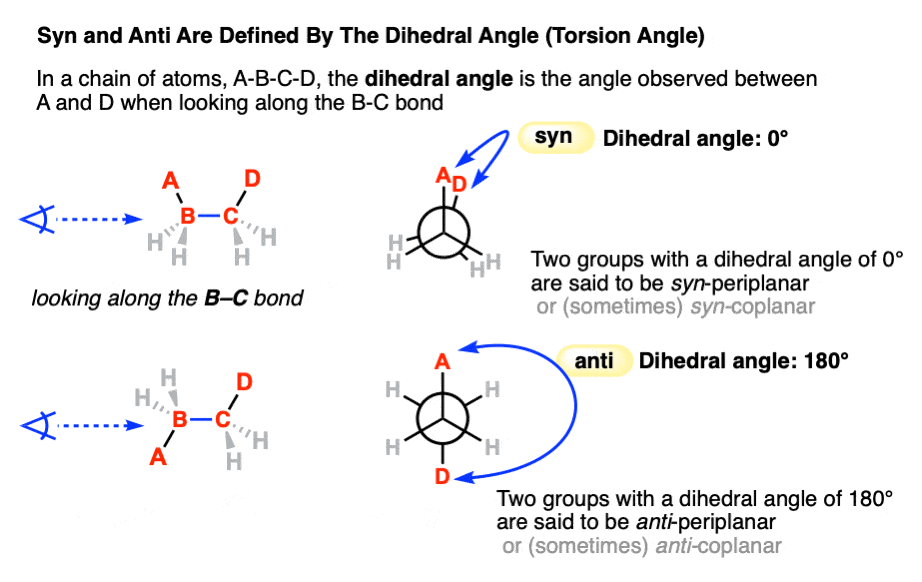
Back in the chapter on conformations, we used the terms syn and anti in Newman projections to refer to the orientation of two groups on adjacent carbons of a carbon-carbon single bond that can rotate freely. (See article: Newman Projection of Butane). When they have a dihedral angle of 0° they are said to be syn. When they have a dihedral angle of 180° they are anti. [Note 2]
Note that syn addition and anti addition refers to the relative orientation of the groups immediately after addition has occurred.
Any subsequent bond rotation about the C-C bond will not change a syn addition product into an anti addition product (or vice versa).
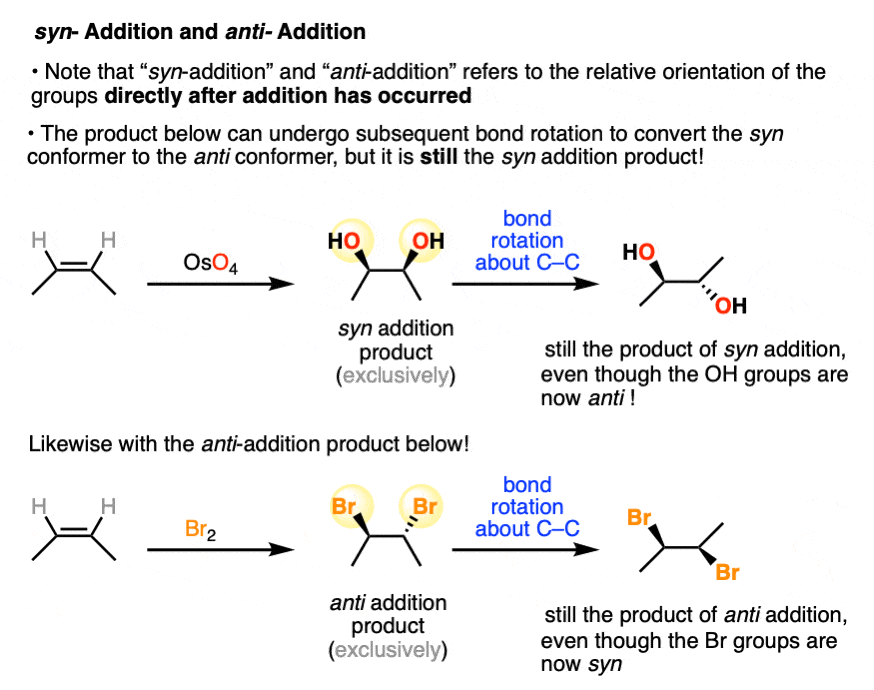
It is tempting to refer to these orientations as “cis” and “trans”, and in some cases the cis and trans products are actually the products. [Note: some of the older chemical literature also uses “cis” and “trans” to refer to syn and anti addition.]
hover to see an example, or click this link.
However, cis and trans should really only be used in situations where the two groups are locked in place and cannot undergo bond rotation (i.e. geometric isomers). Try to avoid using “cis” and “trans” addition for products that can undergo subsequent bond rotation.
5. The Three Categories of Stereoselectivity In Alkene Addition Reactions
The stereoselectivity of alkene addition reactions falls into three categories.
- Reactions that give exclusively syn addition products
- Reactions that give exclusively anti addition products
- Reactions that give a roughly equal mixture of syn and anti addition products.
Whether or not a given reaction gives exclusively syn, anti, or syn+anti products is a piece of information that had to be determined experimentally in each case. You will be told which reaction belongs in which category when we cover each one.
Since you will be expected to draw the products of alkene addition reaction products, knowing which category each reaction falls into is extremely important!
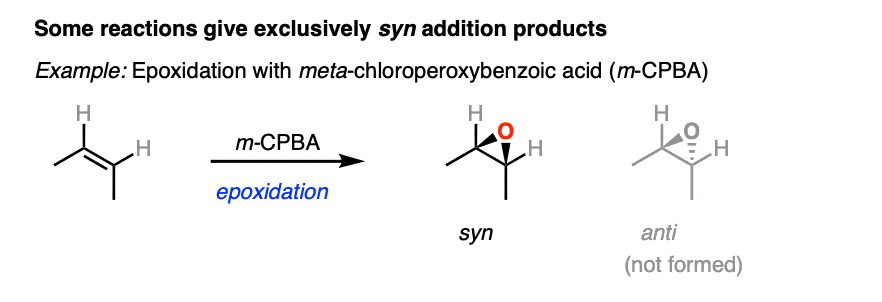
Here’s an example of a reaction that is stereoselective for syn addition products. No products of anti addition are formed.
[See article: What’s A Racemic Mixture?]
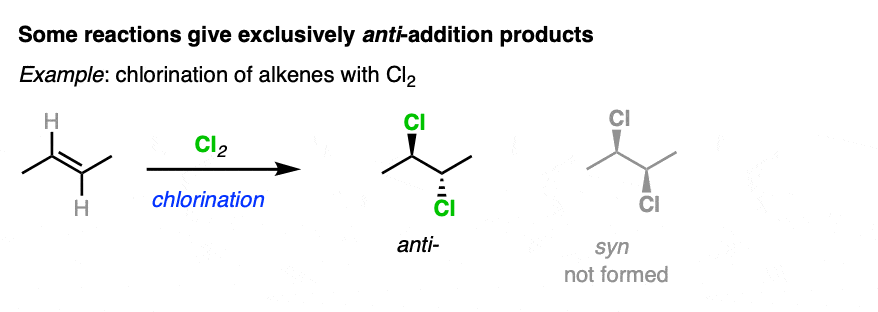
On the other hand, halogenation of alkenes is stereoselective for anti addition products. No syn addition products are formed. [Note 3].
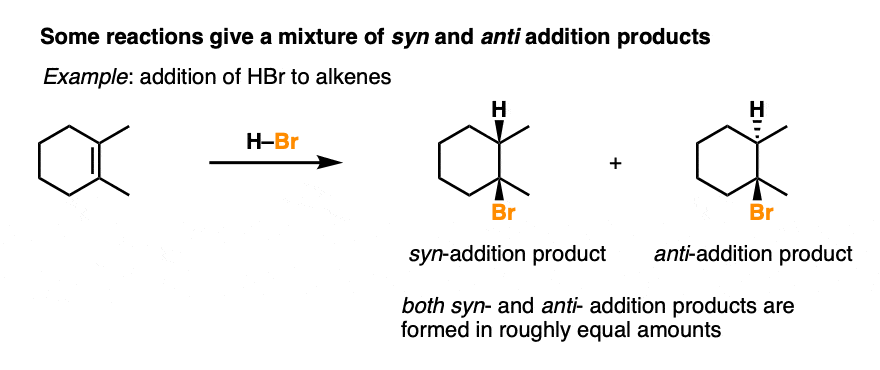
Addition of HX to alkenes is not stereoselective, and gives a roughly equal mixture of syn and anti addition products.
By combining the three patterns of regioselectivity with the three patterns of stereoselectivity, we can build a little 2 × 3 table with all the possibilities.
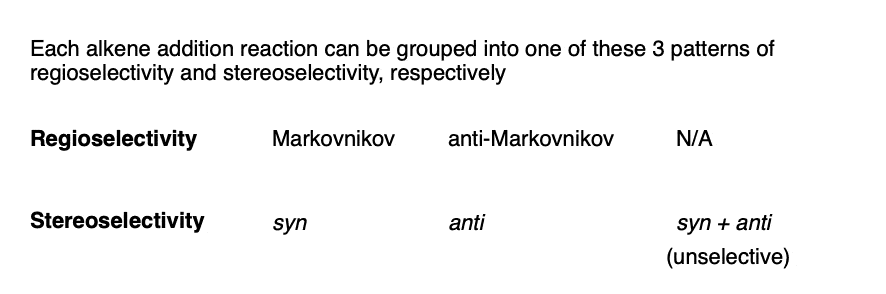
As it turns out, not every one of these possibilities is represented by reactions we will learn.
To skip ahead to the end of the chapter, there will really be three key alkene addition patterns to look out for:
- Reactions that go through carbocation intermediates, which tend to be Markovnikov-selective reactions that give a mix of syn + anti stereochemistry. (See article: Alkene Addition Pattern #1: The Carbocation Pathway).
- Reactions that go through a 3-membered ring intermediate, which tend to be Markovnikov selective (when possible) and give anti addition products. (See article: Alkene Addition Pattern #2: The 3-Membered Ring Pathway).
- Reactions that go through a more or less concerted pathway, which mostly have no regioselectivity (except for hydroboration, which is anti-Markovnikov) and give syn addition products. (See article: Alkene Addition Pattern #3 : The “Concerted Pathway”).
Condensing the reactions of alkenes down into these 3 key buckets will simplify things a lot.
6. Determining Relationships Between Products In Alkene Addition Reactions
Once you have the pattern of bonds that form and break, the regioselectivity, and the stereoselectivity, there’s just one more piece of the puzzle left to address.
Remember that alkenes are flat, and in the vast majority of cases, addition can occur with equal likelihood to either face of the alkene.
Depending on the structure of the alkene, this can result in products that are stereoisomers (enantiomers or diastereomers).
You will need to practice drawing the product obtained for addition to each face, and then compare those two products to determine how they are related.
Remember “enantiomers, diastereomers, or the same?” questions from your chapter on stereochemistry?
Well, we’re going to do this again, in the context of alkene addition reactions. (See article: Enantiomers, Diastereomers or The Same – Two Methods For Solving Problems).
Trust me when I say that this is a very common type of exam question!
This is what makes organic chemistry “special” – concepts from previous chapters (like stereochemistry!) keep recurring and are integrated into the new material.
Let me show you what I mean using addition of H-Br to alkenes as an example.
- In this reaction, a C-C pi bond breaks, and a C-H and C-Br bond forms.
- The reaction has Markovnikov regioselectivity (C-Br forms on most substituted carbon)
- The reaction gives a mixture of syn and anti addition products.
Now let’s explore the products that form when different alkenes undergo addition.
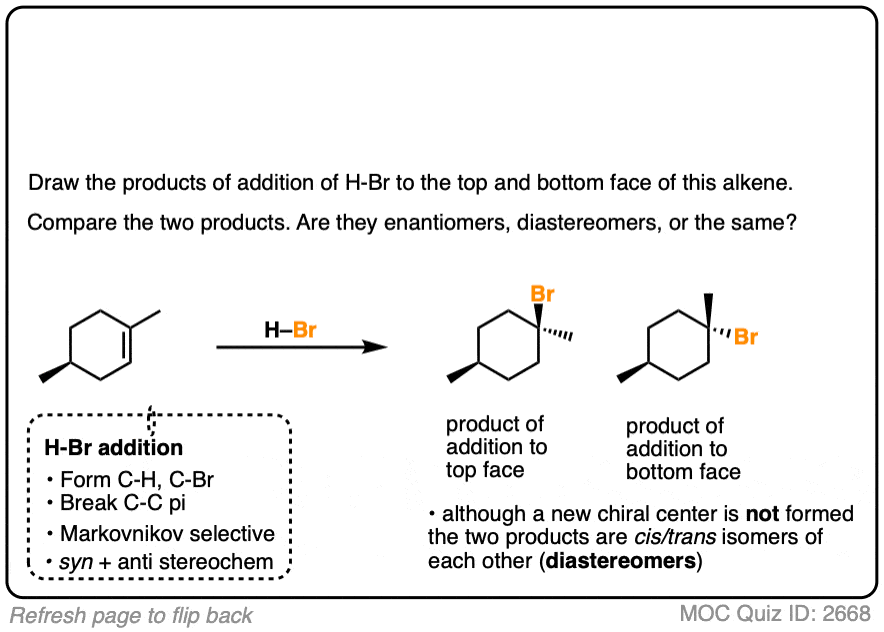
In the addition of H-Br to 1-methylcyclohexene, we can draw the products that are formed when addition happens to the top and bottom face.
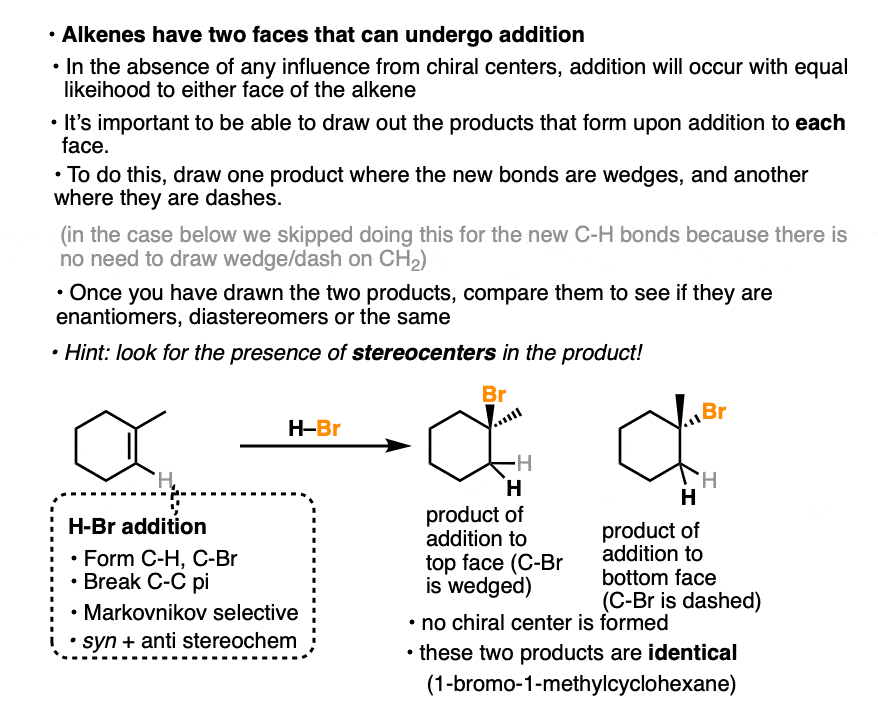
In this case addition to the top face and the bottom face results in the same product (note the absence of any chiral center).
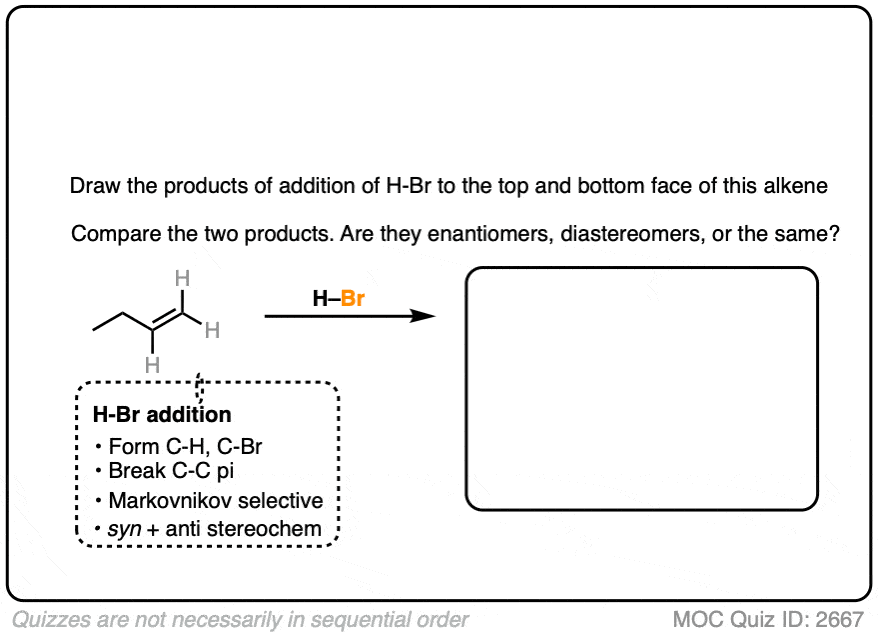
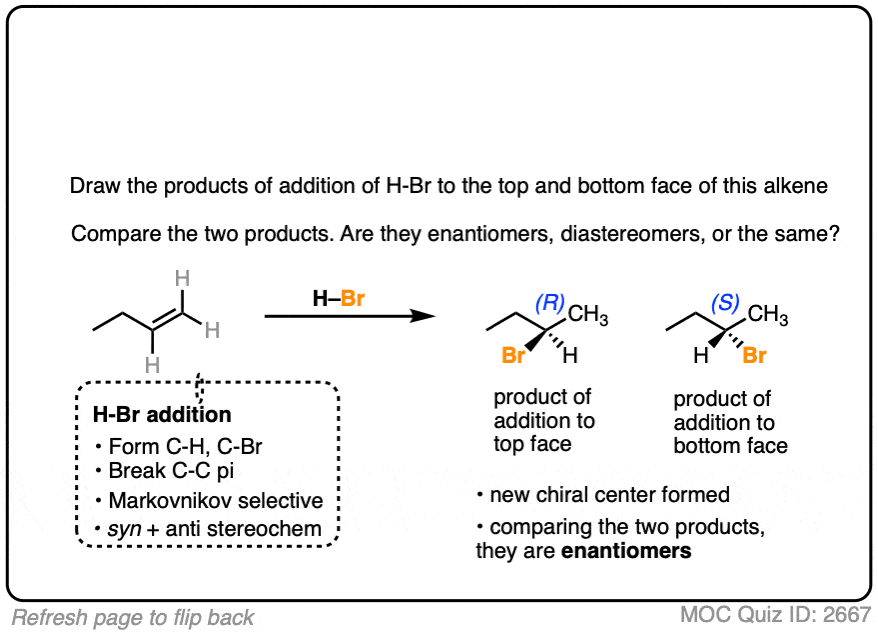
Now let’s try it on 1-butene.
In this case, applying the pattern to both faces gives 2-bromobutane. However, note the formation of a new chiral center. These two products are enantiomers of each other, and since they are formed in equal amounts, they constitute a racemic mixture. (See article: What’s A Racemic Mixture?).
 Click to Flip
Click to Flip
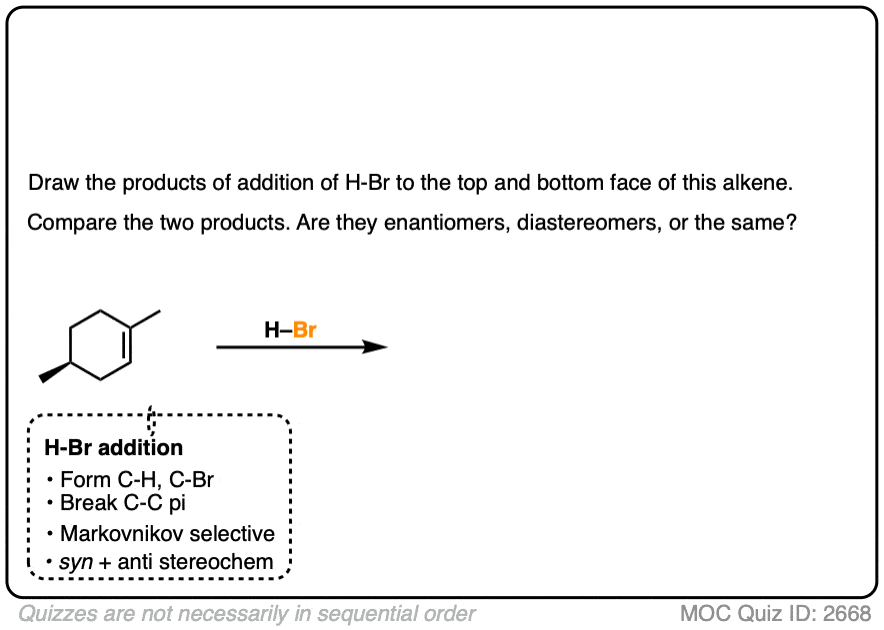
If the starting material has a pre-existing chiral center, look out for the possibility of forming diastereomers.
 Click to Flip
Click to Flip
Is there a simple trick for knowing immediately if a given reaction will give you enantiomers, diastereomers, constitutional isomers or even just the same molecule?
There is no simple trick. Just practice.
However, there’s good news. If you have already answered a lot of “enantiomers, diastereomers, or the same” type questions, then you’re already most of the way toward solving these types of problems.
The key thing here is just applying the pattern for each alkene addition reaction (bonds formed/broken, regioselectivity, stereoselectivity) to each of the two alkene faces, and then comparing the two products.
7. Summary
Lots of concepts covered here, but this lays the groundwork for everything we’ll discuss later in this chapter on alkene addition reactions.
- Every alkene addition reaction involves breaking a C-C pi bond and forming two new bonds to carbon
- There are three key classes of regioselectivity in alkene addition reactions: Markovnikov, anti-Markovnikov, and “none” (where the two bonds are identical).
- There are three key classes of stereoselectivity in alkene addition reactions: syn addition, anti addition, and syn + anti addition (“unselective”).
- With the pattern of bonds formed/broken, regioselectivity, and stereoselectivity in hand you should be able to draw the pattern of any alkene addition reaction.
- Just remember that alkenes are flat and addition can occur to either face of the alkene. Practice drawing out the products that are formed when addition happens to each of the two faces.
- Then compare these two products to determine whether they are enantiomers, diastereomers, or the same
Notes
Note 1. One reaction of alkenes that doesn’t fit into this pattern of addition reactions is oxidative cleavage (e.g. ozonolysis). In this reaction, both the C-C sigma bond and the C-C pi bond are broken, and two new bonds between each carbon and oxygen are formed. (See article: Ozonolysis of Alkenes).
Note 2. Technically, any two substituents with a dihedral angle between +30° and -30° are “syn” and any two substituents with a dihedral angle between +150 and -150° are “anti“.
Note 3. In cases where a very stable carbocation can be formed (such as on a benzylic carbon), the 3-membered halonium ion can open to give a free carbocation, and anti stereoselectivity can be lost. See article: Halogenation of Alkenes.
Quiz Yourself!
[quizzes]
(Advanced) References and Further Reading
[references]
What I dont get is why you can do an elimination reaction of water to get a double bond (catalyzed by H2SO4, E1 mechanism) but you can also get the addition of water to an alkene in acidic conditions. I don’t see how you get 2 different results using the same conditions.
It’s an equilibrium. It depends on whether you have a high concentration of water relative to your alkene (addition) or whether you are actively sequestering water as it’s being formed, taking it out of equilibrium (elimination). Follows Le Chatelier.
This is awesome!