Alkene Reactions
Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes
Last updated: March 21st, 2025 |
Catalytic Hydrogenation of Alkenes With Pd/C (And Friends)
- Alkenes (and alkynes) will undergo addition of hydrogen (H2) in the presence of a metal catalyst such as Pd, Pt, Ni, or Rh.
- These metals are typically finely divided and adsorbed on the surface of a high surface-area material such as activated carbon (most common) or alumina (Al2O3) , hence Pd/C, Pt/C, Rh/Al2O3 and so on. Since these do not dissolve in solution they are called heterogeneous catalysts.
- Since addition results in a new C-H bond at the expense of a C-C bond the oxidation state of carbon decreases. These reactions are commonly referred to as reductions.
- Reduction of alkenes and alkynes generally occurs with high selectivity for syn addition products (See article – Syn and Anti Addition)
- The mechanism (only covered vaguely) involves transfer of hydrogen to alkene on the metal surface
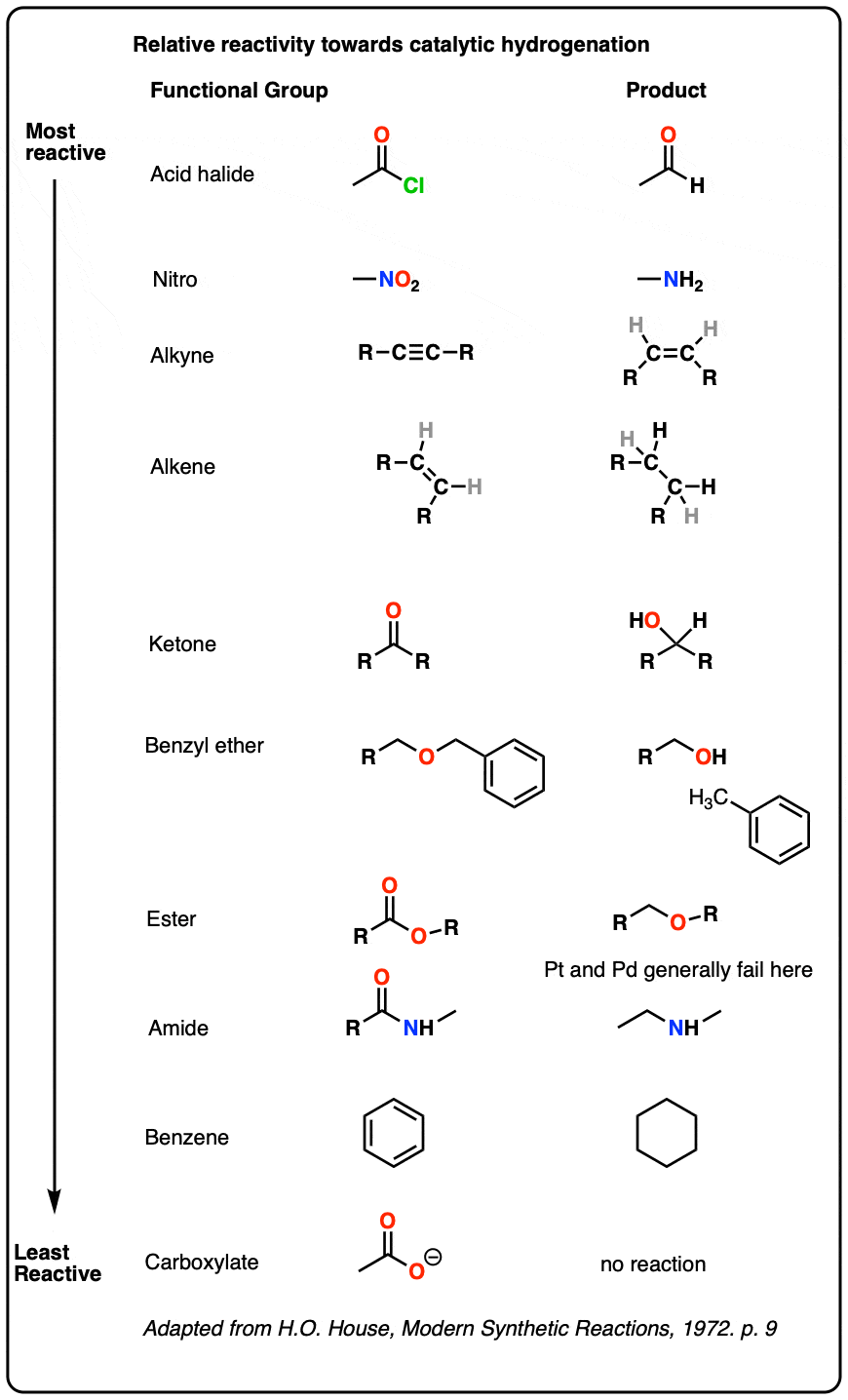
- Alkynes can be hydrogenated selectively over alkenes, which in turn can be hydrogenated selectively over ketones and aldehydes.
- Wilkinson’s catalyst, RhCl(PPh3)3 is a homogeneous catalyst often used for selective hydrogenations.
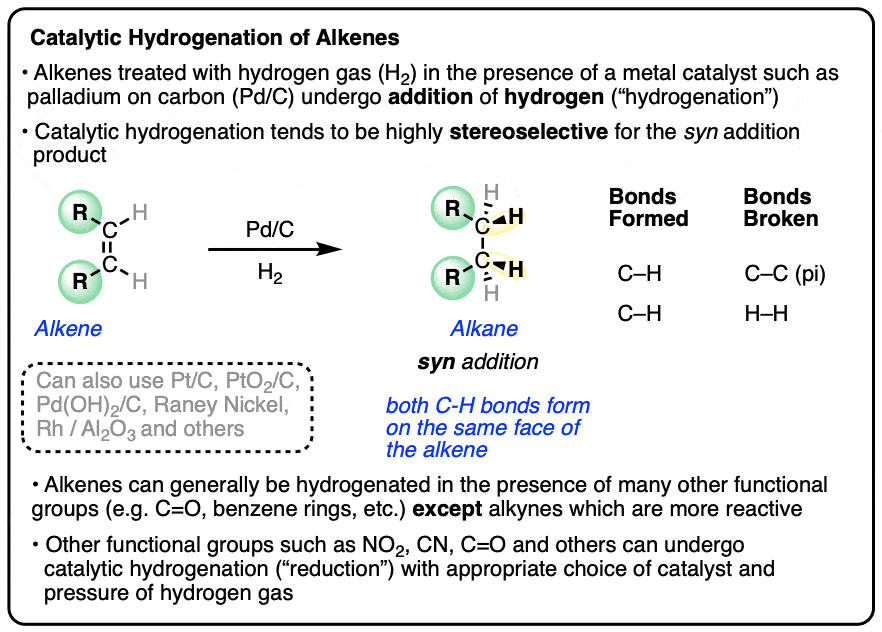
Table of Contents
-
- Catalytic Hydrogenation of Alkenes With Pd, Pt and Other Metals
- Examples of Alkene and Alkyne Hydrogenation
- Hydrogenation is Selective for Syn Addition
- (A Rough Sketch of) A Mechanism for Catalytic Hydrogenation
- Other reactions of Pd/C and H2
- Homogeneous Catalysis using Wilkinsons Catalyst
- Summary
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Catalytic Hydrogenation of Alkenes With Pd, Pt, and Other Metals
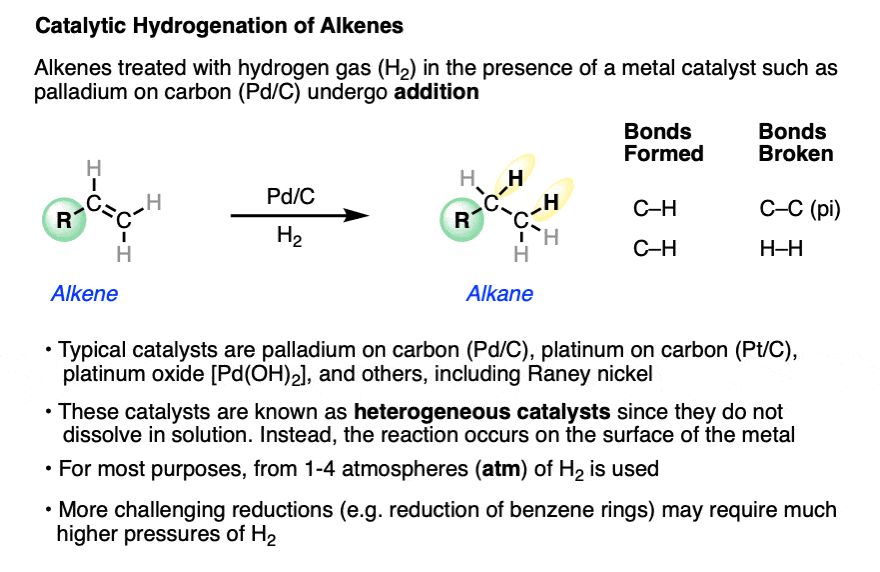
Alkenes (and alkynes) will undergo addition of hydrogen (H2) to C-C pi bonds in the presence of a metal catalyst in a reaction known as “catalytic hydrogenation“.
Discovered in the 1890s by Sabatier, and now used industrially on massive scale for the hydrogenation of vegetable oils. [Note 1]
In this reaction a C-C pi bond (bond dissociation energy [BDE] of about 60 kcal/mol) and a H-H bond (BDE about 104 kcal/mol) are broken, and two C-H sigma bonds (BDE about 98 kcal/mol) are formed, making it exothermic by about 30 kcal/mol.
Since C-H bonds are formed at the expense of C-C bonds, hydrogenation reactions of alkenes and alkynes result in a lowering of the oxidation state of carbon and are often described as “reductions“. See: Oxidation and Reduction in Organic Chemistry.
Despite the significantly greater stability of the products, no reaction between the alkene and H2 typically occurs in the absence of the metal.
The metal catalyst is typically palladium (Pd) or platinum (Pt), although other metals with similar properties such as nickel (Ni) and rhodium (Rh) can also be used. These relatively electron-rich “late” metals react with H2 to form metal-bound hydrides (e.g. H-Pd), which are more reactive towards alkenes than H2 itself. Furthermore, late metals readily adsorb alkenes and other molecules with pi bonds onto their surface. The resulting hydrogenation products, lacking a pi bond, are not bound as strongly to the metal and typically dissociate after hydrogenation, freeing up a site on the metal surface for further reaction.
Think of the surface of Pd/C as kind of like a singles bar, where hydrogen and alkenes (or other organic compounds) meet, react, and leave together. As a catalyst, Pd/C is a lot like a matchmaker who brings couples together, but never gets married himself.
Since the reaction takes place on the metal surface, high surface area is important for reasonable reaction rates. The metal is typically finely divided and adsorbed onto a solid support such as activated carbon or alumina. [Described here]. The result is typically a black powder typically containing 5%-10% metal by weight, e.g. 5% Pd/C or 10% Pt/C.
Since powders do not dissolve in organic solvents, they are referred to as heterogeneous catalysts.
Photo credit. The first time I saw palladium on carbon (Pd/C) and platinum on carbon (Pt/C) I was expecting to see something more traditionally shiny and metallic, rather than a fine black powder.
On small scale, hydrogenation is usually carried out by adding pre-formed, commercially available catalyst (e.g. palladium on carbon, Pd/C) to a solution of the starting material in an inert atmosphere. After H2 is introduced (often via balloon), it’s party time. [Note 2]

Source: Org Prep Daily
Since three phases of matter are involved (gaseous H2, liquid solvent, solid catalyst), vigorous stirring or shaking is necessary for good reaction rates.
Parr shaker apparatus. Source.
When the reaction is complete, the catalyst is separated from the reaction mixture by filtration (note: care must be taken to avoid exposing dry, hydrogenated catalyst to oxygen – fires can result.)
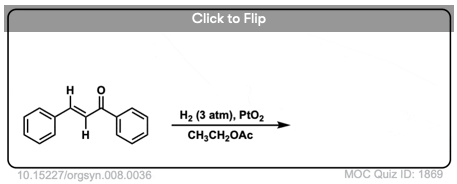
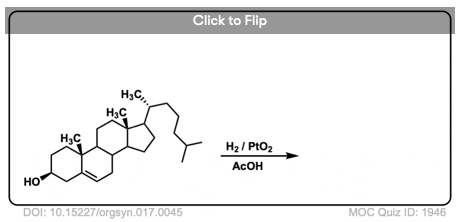
2. Catalytic Hydrogenation of Alkenes: Examples
Both alkenes and alkynes will readily undergo catalytic hydrogenation. Reactions are generally carried out with a molar excess of hydrogen gas, although it is possible to regulate the number of molar equivalents of H2 by using a gas buret or similar device if desired.
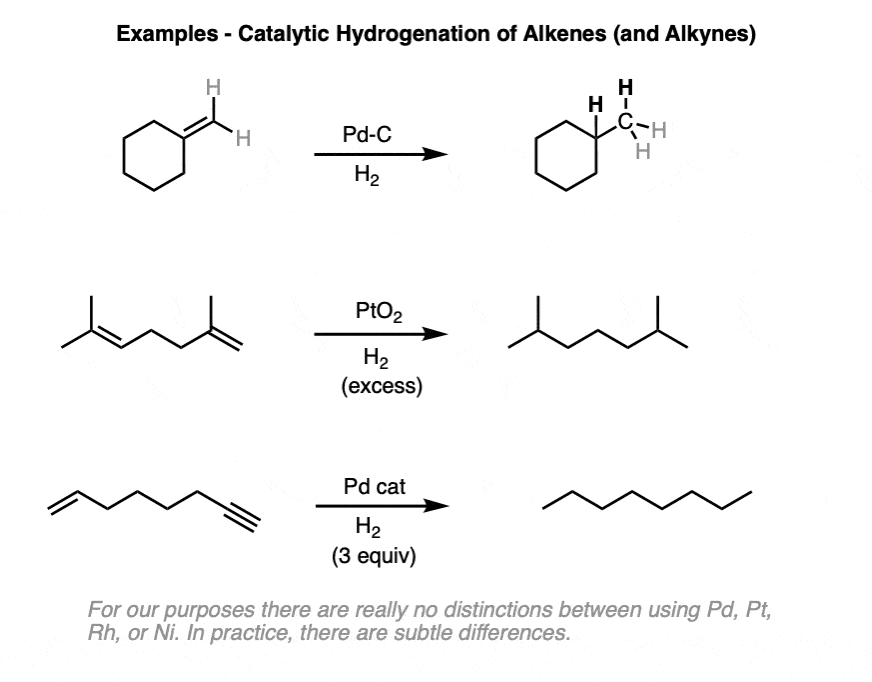
(There is a lot of variety in the specific identity of the metal – for example, one can use palladium (Pd), palladium hydroxide (Pd(OH)2, platinum (Pt), platinum oxide (PtO2), rhodium (Rh), nickel (Ni), Raney Nickel, and more. This covers the most common cases but is not exhaustive! For our purposes (introductory organic chemistry) these can be treated as equivalent. For more specific cases see Note 3. )
The specific pressure of H2 is not always indicated, but is typically 1-4 atmospheres (atm) of hydrogen (15-60 psi). Difficult hydrogenations may require pressure vessels at up to 100 atm.
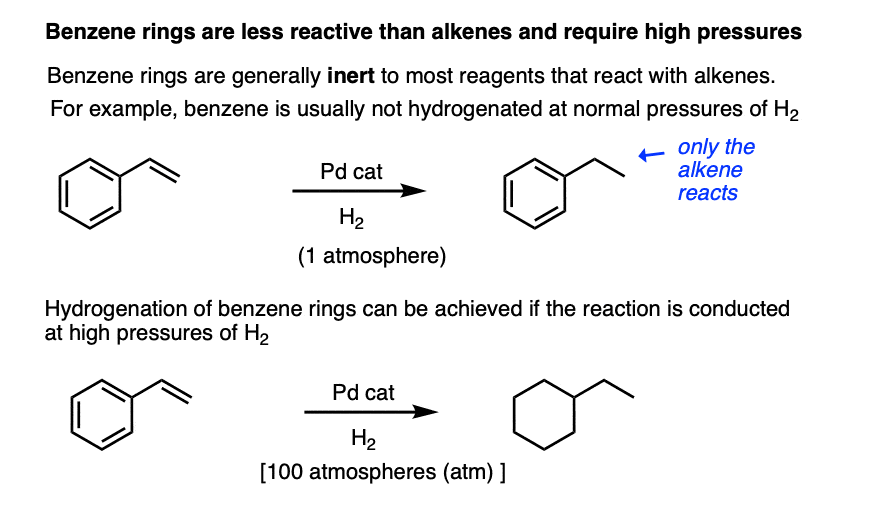
For example, the benzene ring is generally inert towards many reagents that add to alkenes, a topic that we explore in more detail in the chapter on aromaticity (See article – Introduction to Aromaticity).
Hydrogenation of the benzene ring is possible, but typically requires higher pressures of hydrogen gas than do normal alkenes.
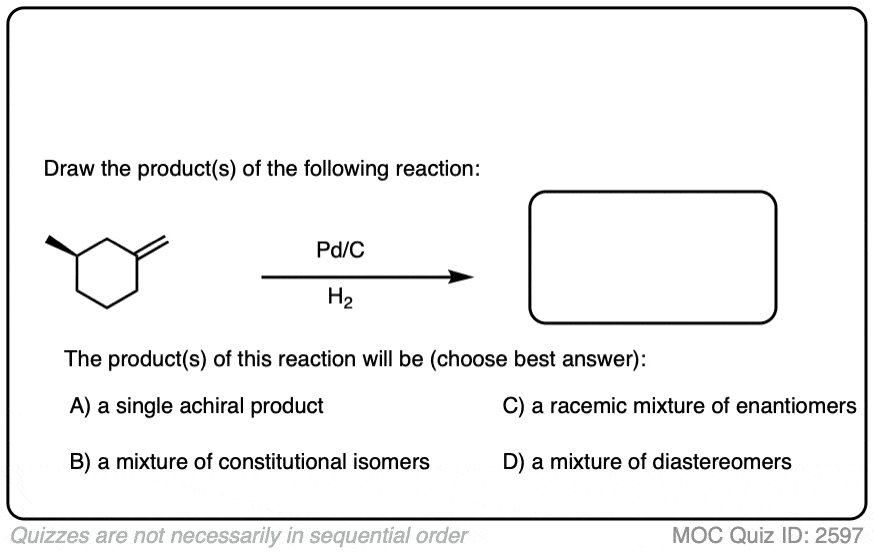
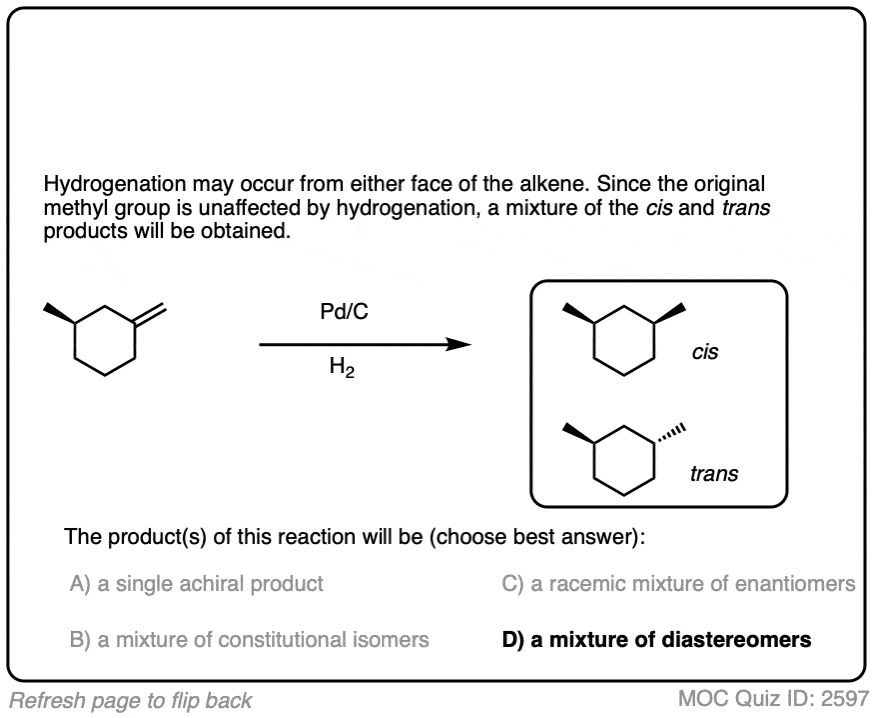
When an alkene is hydrogenated to an alkane, the hybridization at carbon goes from sp2 to sp3. Be alert for the formation of stereoisomers!
 Click to Flip
Click to Flip
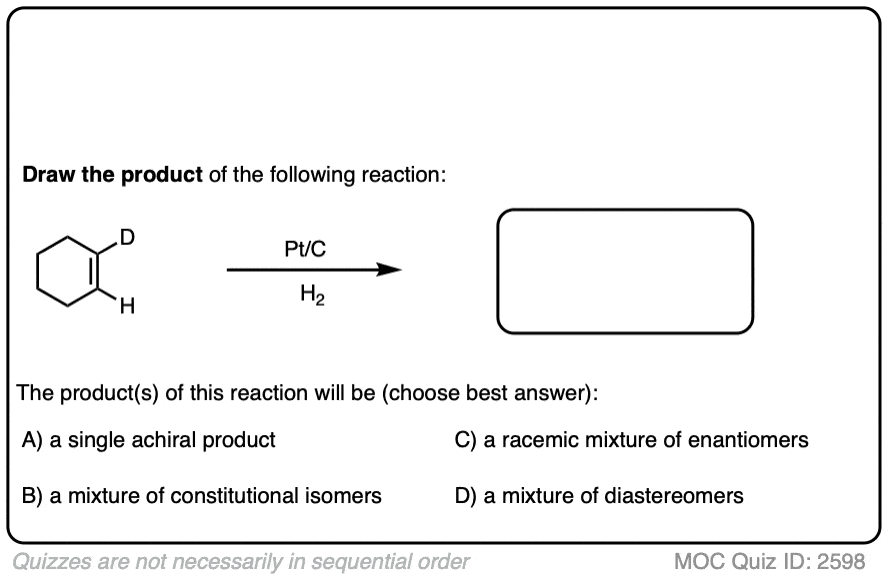
Here is another example in this vein:
 Click to Flip
Click to Flip
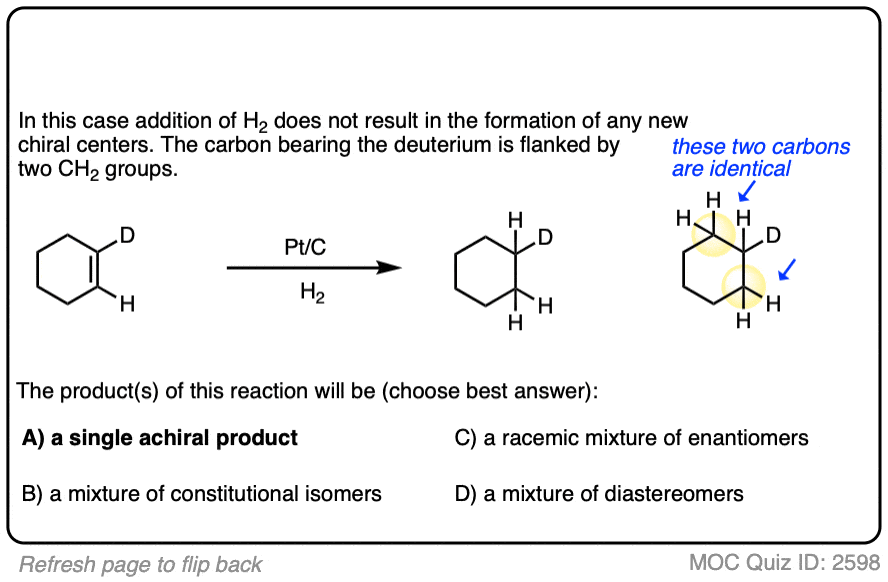
There is no simple formula for answering questions which ask you to say whether a reaction results in enantiomers, diastereomers, constitutional isomers, and so on since it is highly dependent on the structure of the starting alkene. My advice is just to apply the pattern of bonds formed/bonds broken to each reaction and then analyze the relationship between any molecules that form. (See article: Enantiomers, Diastereomers, or The Same? Two Methods for Solving Problems)
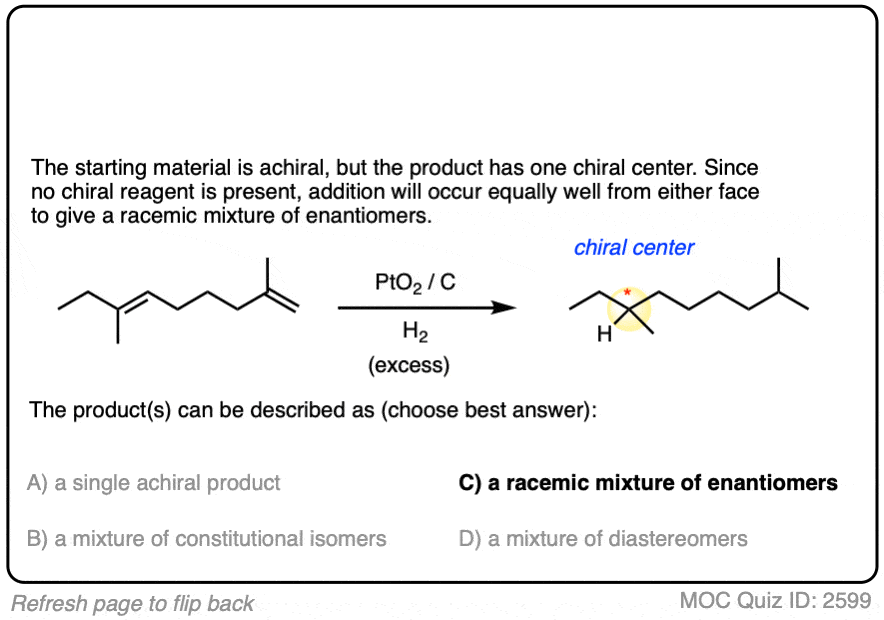
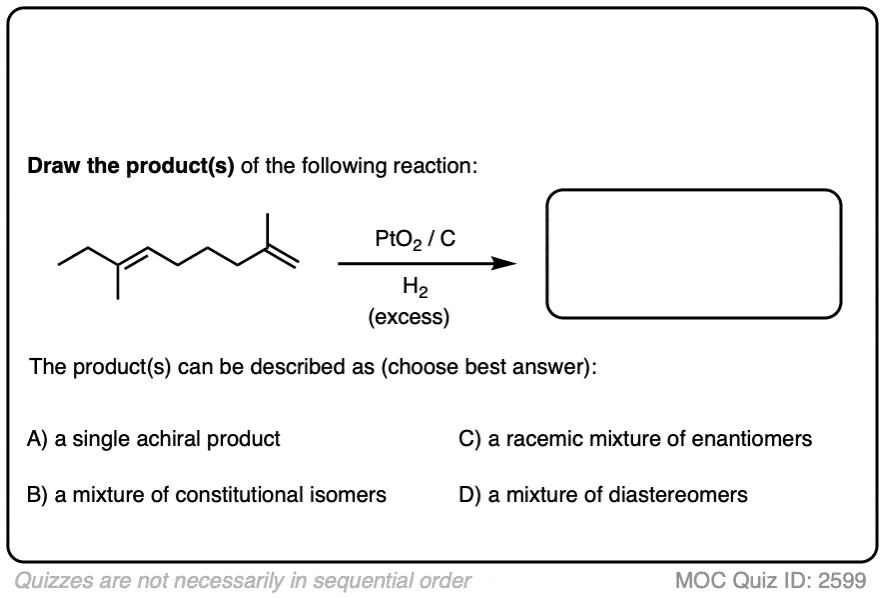 Click to Flip
Click to Flip
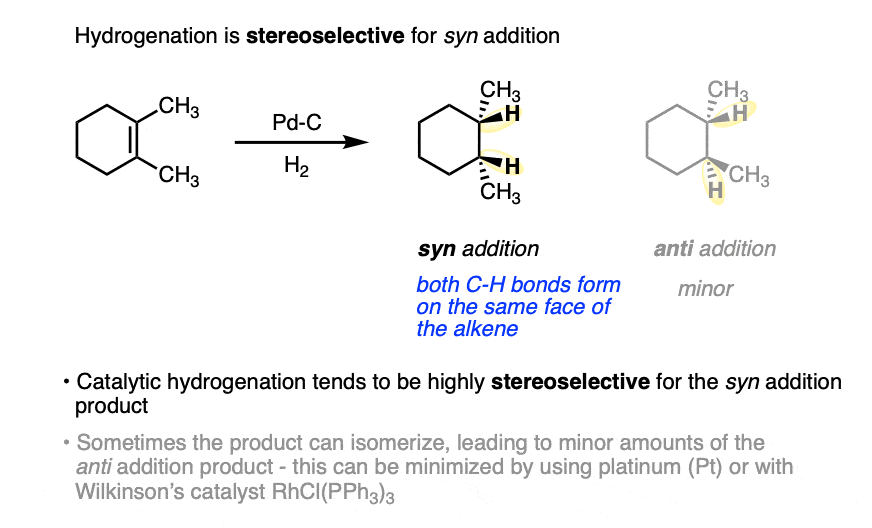
3. Hydrogenation Is Stereoselective for Syn Addition
Hydrogenation is generally a stereoselective reaction where the two C-H bonds form on the same face of the alkene.
This is known as syn addition (in contrast to anti addition where the two new bonds form on opposite faces of the alkene).
For example, the major product in the hydrogenation of 1,2-dimethylcyclohexene is cis-1,2-dimethylcyclohexane.
In general, catalytic hydrogenation is highly stereoselective for syn addition. [Note 4]
In practice, particularly with Pd (and less so with Pt) a significant amount of double bond migration can occur before hydrogenation is completed. A related process is responsible for the formation of trans fats in partial hydrogenation of vegetable oils, for example. [Note 5]
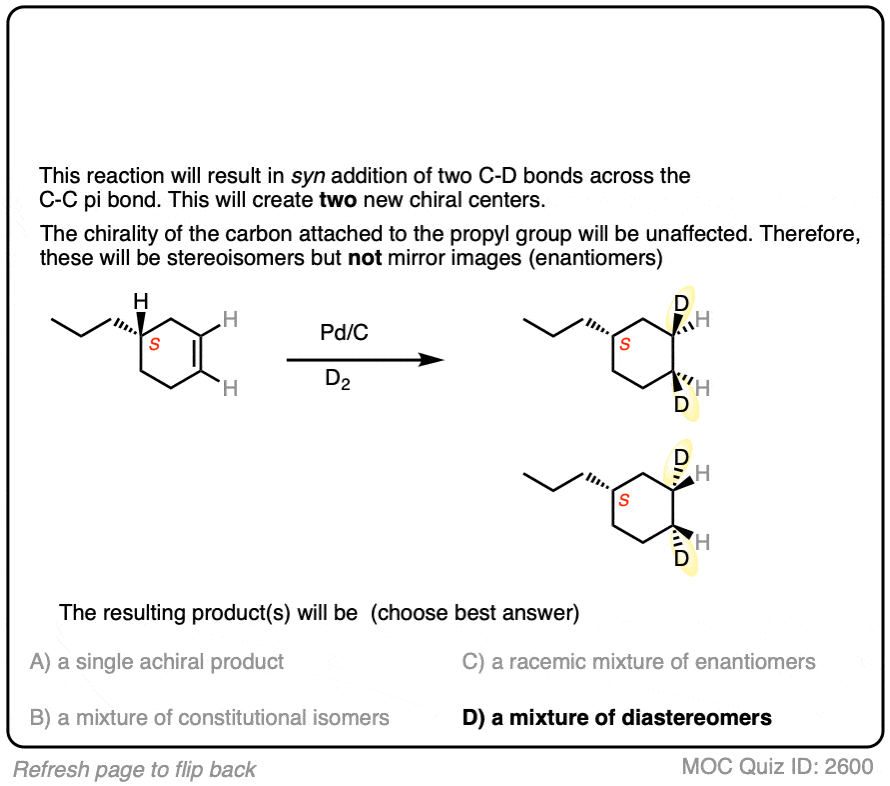
Deuterium (D2) can be used in place of hydrogen to give isotopically labelled products (recall that deuterium is the heavy isotope of hydrogen). The reaction is otherwise identical in all respects, including a preference for syn addition.
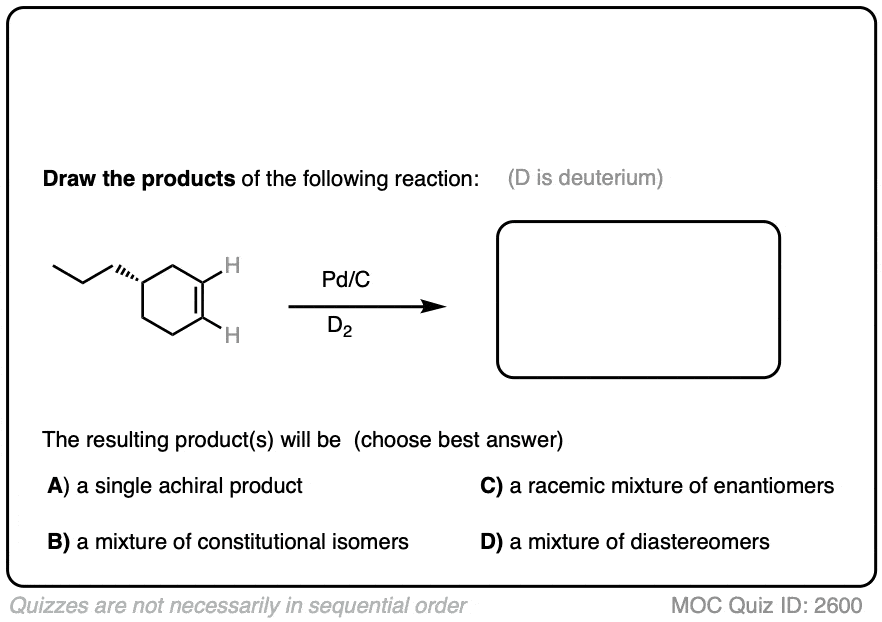 Click to Flip
Click to Flip
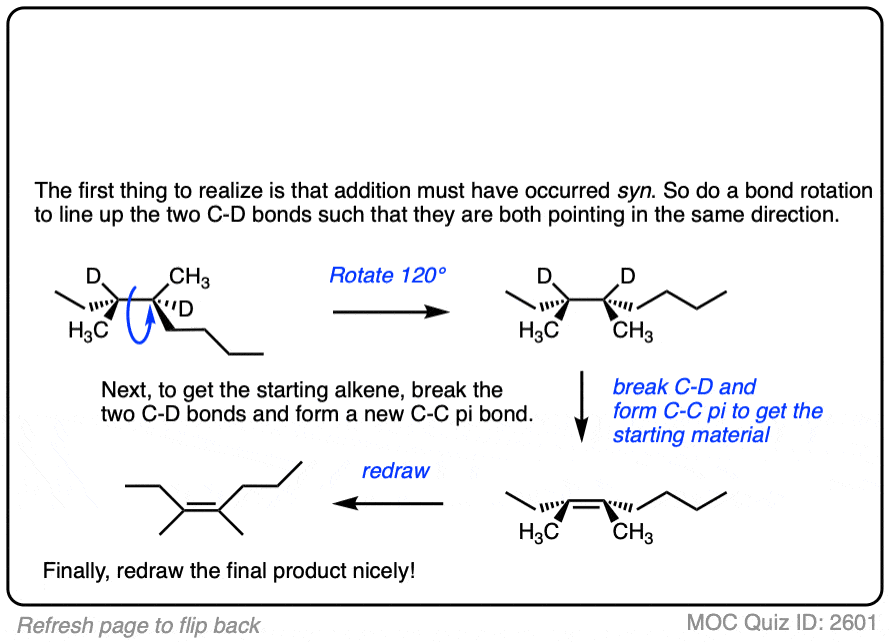
See if you can work backwards from the final product to the original alkene starting material:
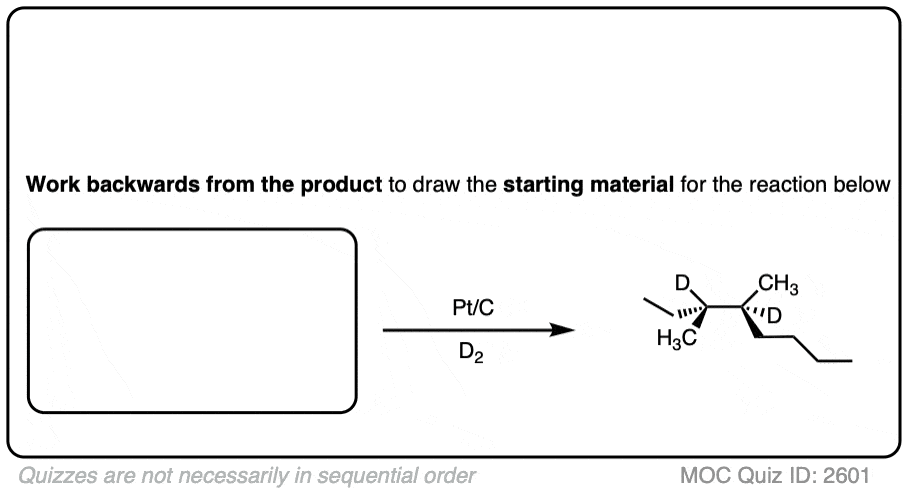 Click to Flip
Click to Flip
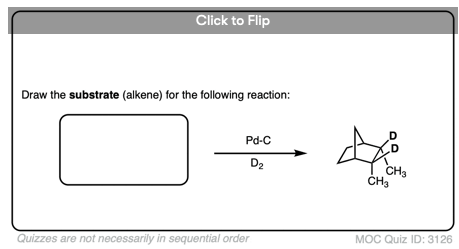
Be alert that in some molecules, the two faces of the alkene are not equally accessible to reagents. In these cases the less hindered side of the alkene will end up bound to the metal.
For example in the bicyclic molecule below, the top face is more accessible since it is only blocked by a one carbon (CH2) bridge, whereas bottom approach is blocked by a two-carbon bridge.
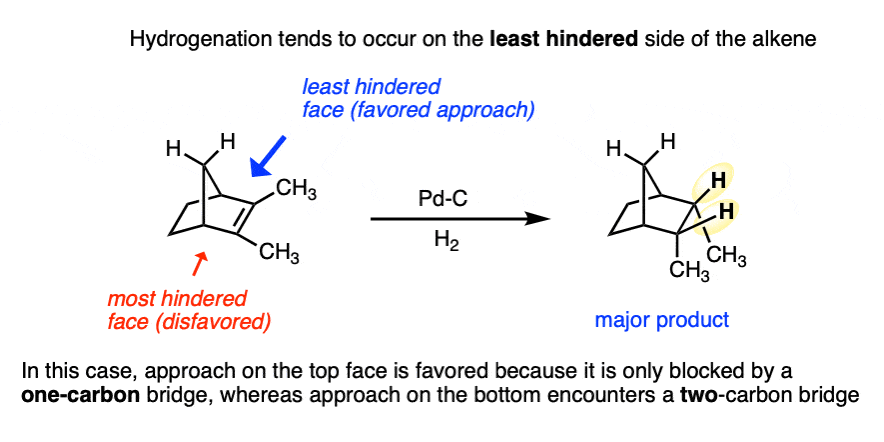
4. Catalytic Hydrogenation – A Rough Mechanism
The mechanism of catalytic hydrogenation is unusual for us in that it’s not a typical arrow-pushing mechanism.
It happens on a metal surface and all the specific details of how hydrogenation happens on that metal surface haven’t been completely worked out.[Note 6]
As Wolfgang Pauli said; “God made the bulk; the surface was invented by the devil”.
Furthermore, it involves some mechanisms of transition metals that aren’t really taught fully in introductory organic chemistry classes.
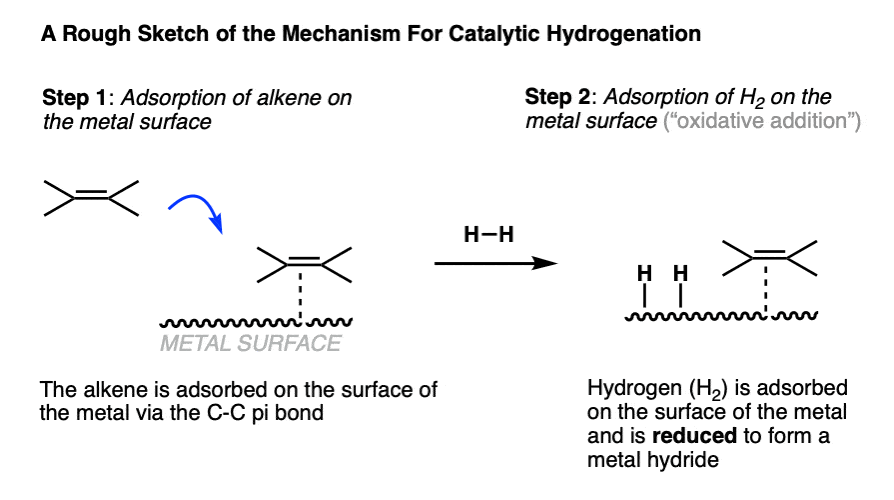
The first two steps of catalytic hydrogenation involve coordination of the alkene and of H2 to the metal surface.
- The starting alkene (or alkyne, or other starting material) is typically adsorbed on the surface of the metal through its pi-bond.
- When hydrogen gas (H2) is introduced to the metal, it is converted into metal hydrides that reside on the surface. (Note 7 )
- With the hydride and alkene in close proximity, a C-H bond forms and the C-C pi bond breaks, resulting in a metal-carbon bond. (Note 8)
- Finally, the second carbon-hydrogen bond forms, resulting in an alkane. [Note 9] Without any pi bond, the molecule dissociates.
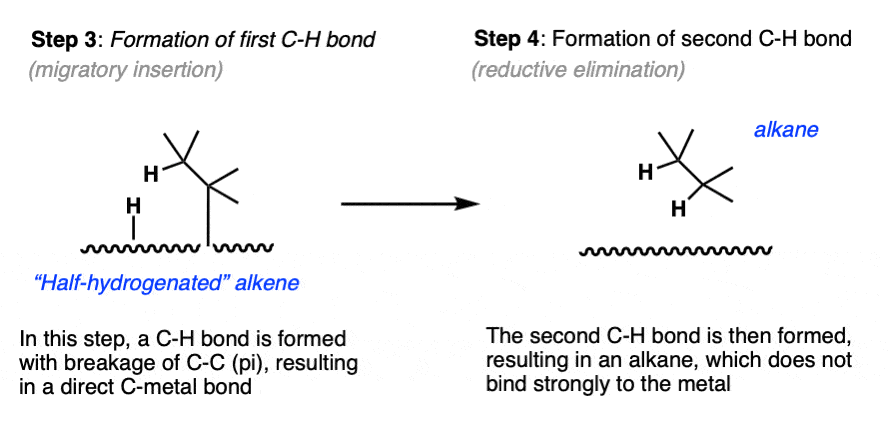
Note the two atoms of H2 are not introduced at exactly the same time!
5. Alkenes vs. Alkynes vs. C=O and Other Functional Groups
Alkynes are even more reactive towards hydrogenation than alkenes, and can typically be reduced to alkenes without affecting the other alkenes in the molecule.
The best way to do this is generally with Lindlar’s Catalyst (See article – Lindlar’s catalyst) or with sodium in ammonia, both of which only reduce alkynes.
It can also be done with Pd, usually something like barium sulfate. BaSO4.
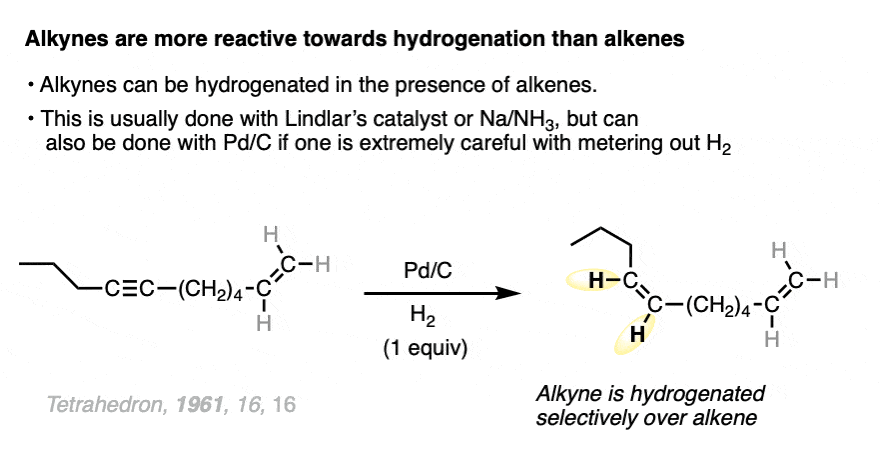
When two or more alkenes are present on the same molecule, it can be possible to hydrogenate one alkene without reducing the other if care is taken to introduce only one mole of H2.
It’s usually the least substituted alkene that is hydrogenated first, because it’s more accessible to the metal catalyst.
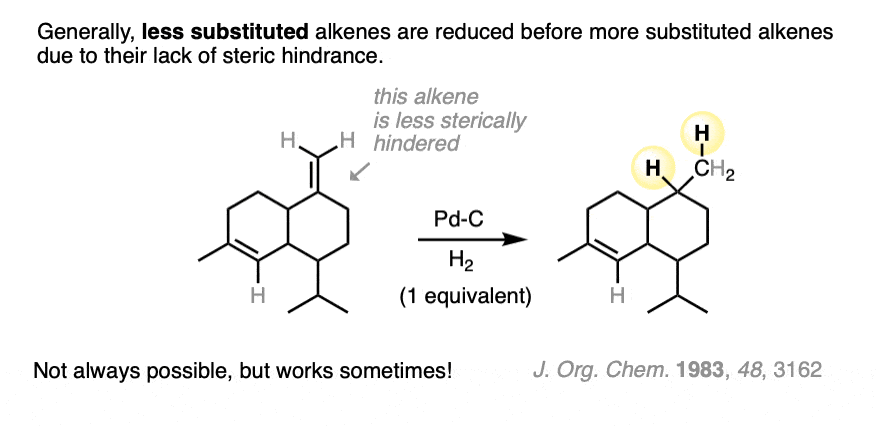
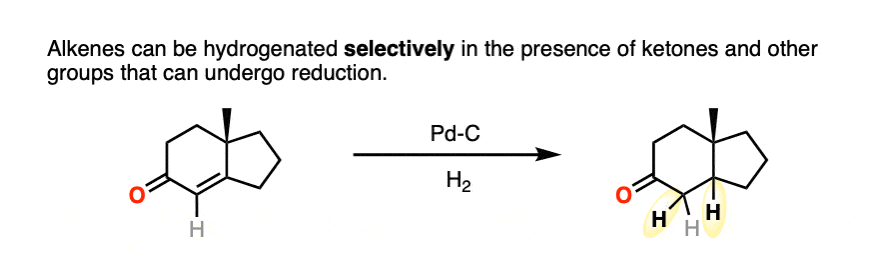
Ketones and aldehydes are even less reactive toward catalytic hydrogenation than are alkenes or alkynes. It’s very possible to hydrogenate a double bond without aldehyde or ketone being affected.
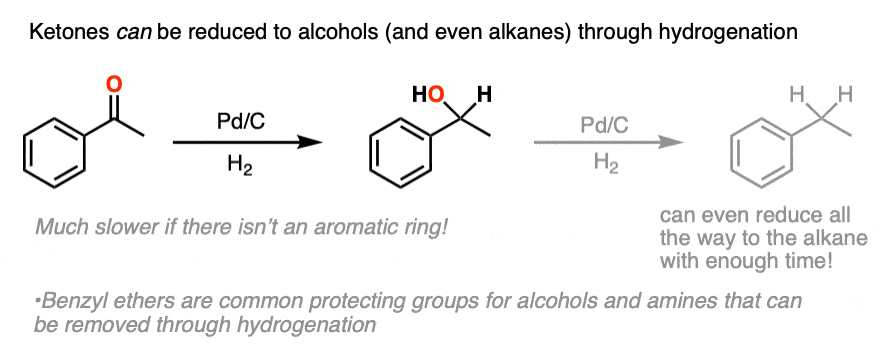
When pushed, ketones can be converted to alcohols (C-OH) or even alkanes (CH2) ; this topic usually comes up when we cover the reactions of aromatic compounds.
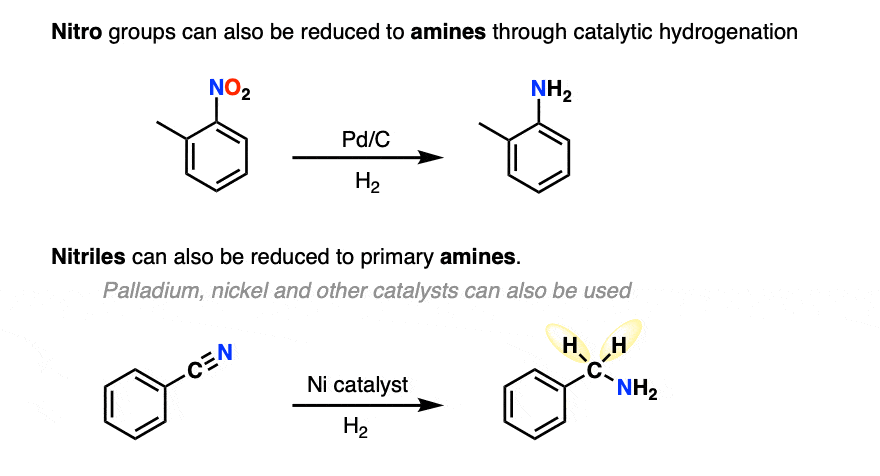
Catalytic hydrogenation can also be used to convert nitro groups and nitriles to amines (although to be fair this is typically covered much later in the course!)
6. Homogeneous Catalysis With Wilkinson’s Catalyst
An example of a homogeneous hydrogenation catalyst is Wilkinson’s catalyst, RhCl(PPh3)3 . One advantage of carrying out hydrogenations with Wilkinson’s catalyst is a lack of the scrambling that can sometimes occur with Pd.
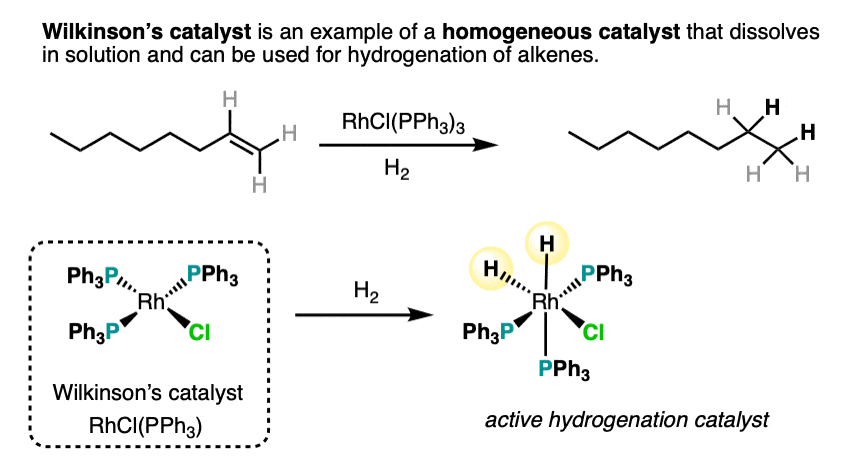
The reactions of transition metal catalysts such as Wilkinson’s catalyst are generally beyond what we cover in introductory organic chemistry as they involve mechanisms such as oxidative addition, migratory insertion, reductive elimination and others that are not necessarily difficult to learn, but require a fair amount of background in inorganic chemistry to understand.
For advanced students: these notes from Prof. M.C. White (UIUC) are a truly excellent place to start. Wilkinson’s Catalyst
7. Summary
Alkenes (and alkynes) can be converted to alkanes through treatment with H2 and a metal catalyst such as Pd, Pt, Ni, or Rh. For our purposes these catalysts are essentially the same, although there are some subtle differences. The metal is usually adsorbed on a high surface area material (such as activated carbon) and filtered off after use.
This is considered a reduction since a new C-H bond is formed at the expense of a C-C bond and the oxidation state of carbon is reduced.
For our purposes the reaction is considered concerted and selective for syn addition.
Pd/C can also be used for reduction of nitro groups (NO2) and even CN and aromatic rings. For difficult reductions higher pressures of H2 and higher temperatures may be used.
An example of a homogeneous catalyst for hydrogenation is Wilkinson’s catalyst, RhCl(PPh3)3. This reagent is completely stereospecific.,
Notes
Note 1. Unsaturated fats contain double bonds and are commonly found in butter, among many other natural sources. The problem with unsaturated fats is that they slowly react with molecular oxygen. The C-H bonds on the carbons adjacent to the double bond (the “allylic” positions) are unusually weak, since removal of H by O2 in homolytic fashion gives a resonance stabilized allylic radical. This then combines with the O-OH radical to give a hydroperoxide, which in the case of butter eventually breaks down to butyric acid, the smell of rancid butter.
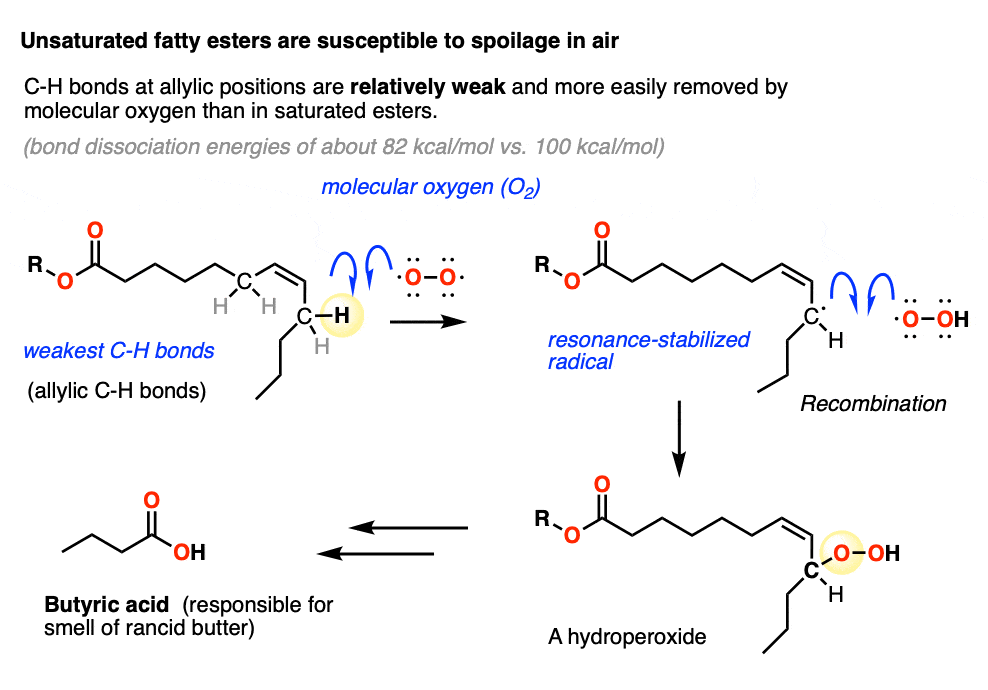
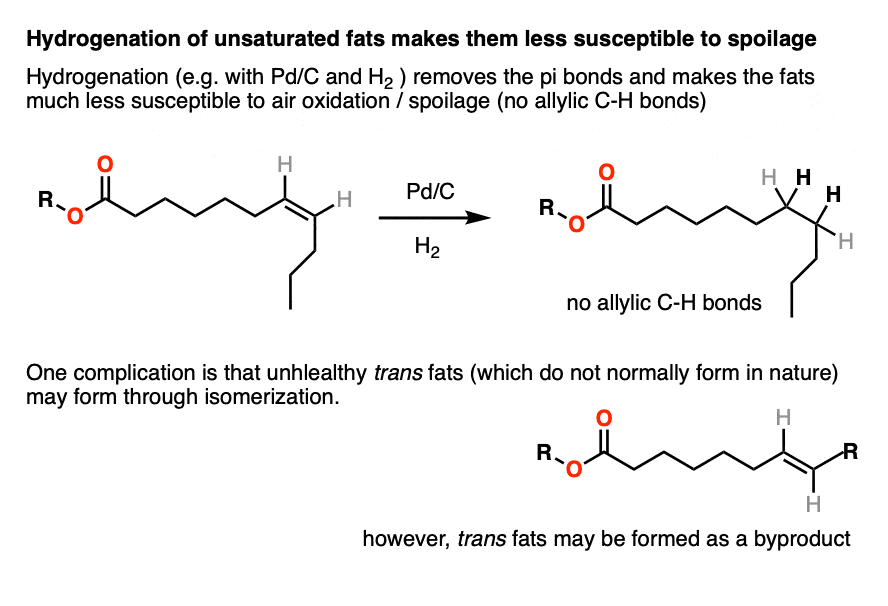
Hydrogenation of unsaturated fats gives saturated fats, which are much more resistant to homolytic removal of C-H by oxygen. They have very long shelf lives and do not typically spoil.
The double bonds in naturally occurring saturated fats are cis. One problem with hydrogenation is that it can result in isomerization of the double bond to give trans fats, which are very bad.
Note 2. Some important safety considerations with Pd/C. Hydrogen gas is generally introduced last. If catalyst must be introduced last, make sure the flask has been completely purged of oxygen. Don’t add finely divided metal catalyst to a flask containing hydrogen and oxygen; fires can result this way.
Raney nickel comes with H2 already adsorbed to the metal surface. In the presence of oxygen, it’s important to keep the catalyst wet to avoid fires.
Be careful when filtering off the metal catalyst at the end of the reaction; the finely divided nickel still contains adsorbed hydrogen, and sucking a bunch of air through the catalyst bed can result in fires. Note the red glowing catalyst in the video below!
This is from filtration of Raney Nickel. Source: Named Reactions In Organic Chemistry Youtube Channel
Note 3. Which catalyst should you use? For our purposes, Pd/C is good for pretty much everything, but some textbooks (and instructors) have quirks. For the curious, a good “which catalyst should you use” discussion for many different reactions can be found here (page 43).
A useful table of relative reactivities of various functional groups toward Pd/C puts acid halides at the top and aromatic rings at the bottom. The table below is adapted from House who in turn adopted it from Rylander.
Note 4. While hydrogenation of alkenes is usually selective for cis addition, this is not always the case. One prominent example of the non-stereospecificty of this reaction can be found in the hydrogenation of the tetrasubstituted alkene below.
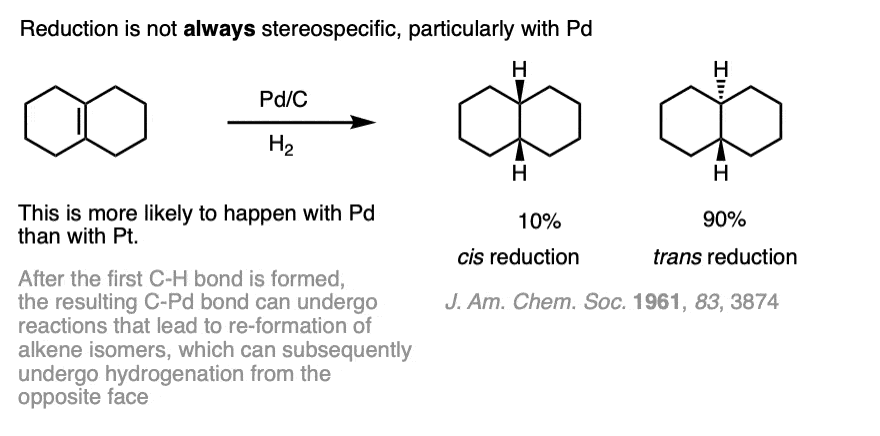
This lack of selectivity for syn addition arises from the half-hydrogenated product which has a C-Pd bond. Considerable isomerization can occur when Pd is used. Isomerization can be greatly minimized by using platinum (Pt).
Note 5. Isomerization of half-hydrogenated product can lead to formation of anti addition products. These isomerizations can be minimized by using platinum.
Note 6. The first rough proposal for catalytic hydrogenation was published in 1934 [Ref] and this has largely stood the test of time. More modern techniques have been helpful for quantifying mechanism, since Pd can vary widely across vendors depending on what method is used for its preparation.
Note 7. This step is called, “oxidative addition”. The Pd(0) reacts with H–H to form H-Pd-H. Palladium is oxidized from the zero oxidation state to the Pd(II) oxidation state, and hydrogen is reduced from the zero oxidation state to the (-1) oxidation state.
Note 8. This step, called, “migratory insertion”, results in formation of a new C-H bond with breakage of C-C (pi) and formation of a new C-Pd bond.
Note 9. This step is called, “reductive elimination”. The C-H bond is formed, and palladium goes from the Pd(II) oxidation state back to Pd(0). It is now ready to re-enter the catalytic cycle.
Quiz Yourself!

Become a MOC member to see the clickable quiz with answers on the back.

Become a MOC member to see the clickable quiz with answers on the back.
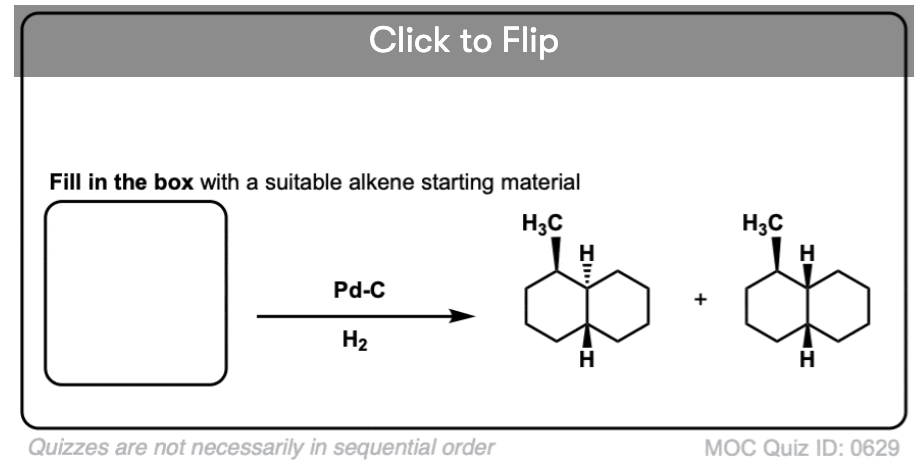
Become a MOC member to see the clickable quiz with answers on the back.
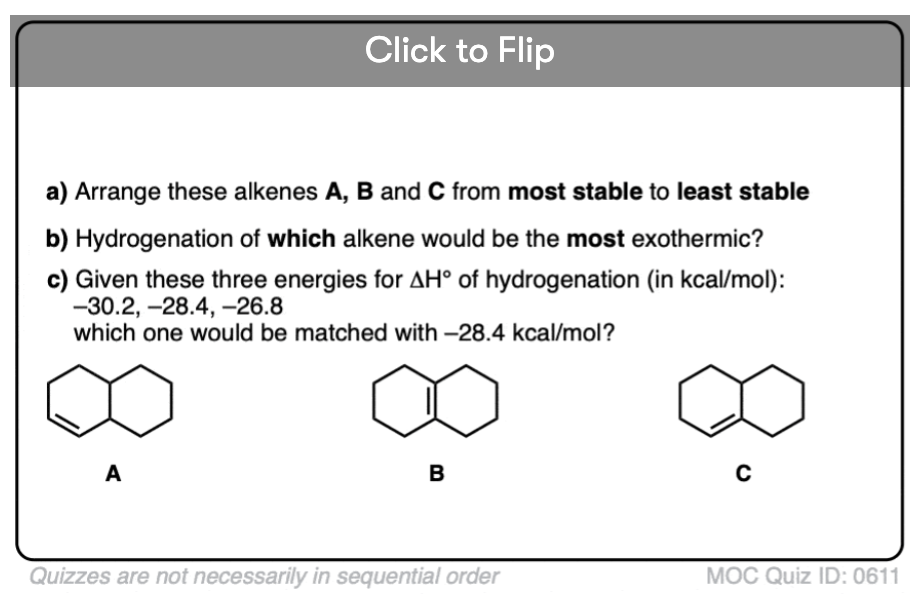
Become a MOC member to see the clickable quiz with answers on the back.
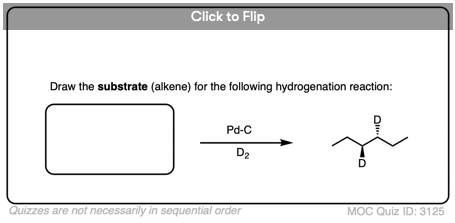
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
- A useful reference for this article has been Hydrogenation Methods by Rylander (1985). Available for loan on the Internet Archive, here. Aldrichimica Acta has a good summary on which catalyst to use for which reaction. Page 43
- Modern Synthetic Reactions [link] by H.O. House has a useful first chapter overview.
- How Not To Do It: Hydrogen Balloons
- The Method of Direct Hydrogenation by Catalysis
Paul Sabatier
Nobel Lecture, December 11, 1912
https://www.nobelprize.org/prizes/chemistry/1912/sabatier/lecture/
The French chemist Paul Sabatier is considered the ‘father’ of hydrogenation, and received the Nobel Prize in 1912 for his work. This is his Nobel Lecture, describing the path to discovery and his contributions to chemistry.
“When a stream of ethylene is directed on to nickel, cobalt or iron, which has been freshly reduced and kept in the region of 300°C, intense incandescence of the metal with deposition of large quantities of carbon due to breakdown of the ethylene occurs. However, the gas which leaves the apparatus is not hydrogen but consists mainly of ethane.” - APPARATUS FOR CATALYTIC REDUCTION
Roger Adams and V. Voorhees
Org. Synth. 1928, 8, 10
DOI: 10.15227/orgsyn.008.0010
This describes in detail how to build a reactor for catalytic hydrogenation. Nowadays these can be commercially purchased – the “Parr reactor” is very common. - Exchange reactions of hydrogen on metallic catalysts
Iurô Horiuti and M. Polanyi
Trans. Faraday Soc., 1934, 30, 1164-1172
DOI: 10.1039/TF9343001164
Originally postulated mechanism for catalytic hydrogenation on a metal surface, which has held up pretty well. - PALLADIUM CATALYSTS
Ralph Mozingo
Org. Synth. 1946, 26, 77
DOI: 10.15227/orgsyn.026.0077
Detailed procedures of how to prepare palladium on barium sulfate (Pd/BaSO4), palladium chloride on carbon, and both 5% and 10% palladium on carbon (Pd/C), from Organic Syntheses, a compendium of reliable methods for the preparation of organic compounds. - The Hydrogenation of Cyclohexenes over Platinum Oxide
James-Frederick Sauvage, Robert H. Baker, and Allen S. Hussey
Journal of the American Chemical Society 1960, 82 (23), 6090-6095
DOI: 10.1021/ja01508a029
Catalytic hydrogenation usually proceeds with addition of both hydrogen atoms to the same face of the double bond (syn addition). Adsorption to the catalyst surface normally involves the less sterically hindered face of the double bond, and this is seen in this paper. -
The Stereochemistry of the Hydrogenation of Cycloolefins on Supported Palladium Catalysts
Samuel Siegel and Gerard V. SmithJournal of the American Chemical Society 1960 82 (23), 6087-6090
DOI: 10.1021/ja01508a028
One side-reaction that occasionally occurs with Pd-C is isomerization of adjacent stereocenters, as seen in this paper. Isomerization is less likely when using platinum as the catalyst. - The Reaction of Sodium Borohydride with Nickel Acetate in Ethanol Solution–A Highly Selective Nickel Hydrogenation Catalyst
Herbert C. Brown and Charles A. Brown
Journal of the American Chemical Society 1963, 85 (7), 1005-1006
DOI: 10.1021/ja00890a041
Catalytic hydrogenations are usually very clean reactions with little byproduct formation, but careful studies reveal that sometimes double bond migration can take place in competition with reduction. In this case, hydrogenation of 1-pentene over ‘P-2 nickel boride’ (nickel acetate reduced with NaBH4) is accompanied by some isomerization to both E– and Z-2-pentene. - Low-temperature hydrogenation over borohydride-reduced catalysts. A new convenient procedure for improving the selectivity of reduction
Charles Allan Brown
Journal of the American Chemical Society 1969, 91 (21), 5901-5902
DOI: 10.1021/ja01049a050
This paper demonstrates that 1,2-dimethylcyclohexene can be reduced with a preferential syn addition from the less hindered side over Pt under H2. - Stereochemical Control of Reductions. 9. Haptophilicity Studies with 1,1-Disubstituted 2-Methyleneacenaphthenes
Hugh W. Thompson and Shaker Y. Rashid
The Journal of Organic Chemistry 2002, 67 (9), 2813-2825
DOI: 10.1021/jo010633b
Functional groups can also exert directing effects in catalytic hydrogenation. The substituent can interact with the catalyst surface and direct hydrogenation towards the same side that is closest to it. - Defining the Qualities of High-Quality Palladium on Carbon Catalysts for Hydrogenolysis
Conor J. Crawford, Yan Qiao, Yequn Liu, Dongmei Huang, Wenjun Yan, Peter H. Seeberger, Stefan Oscarson, and Shuai Chen
Organic Process Research & Development 2021 25 (7), 1573-1578
DOI: 10.1021/acs.oprd.0c00536
Very in depth study on the active catalyst for hydrogenolysis of O-benzyl groups. - Double bond migration and racemization during the hydrogenation of olefins
Journal of Catalysis, 1963, 2 (6) 498-505
DOI: 10.1016/0021-9517(63)90005-8
From the abstract: “Double bond migration and racemization during the hydrogenation of several optically active alkenes have been studied. Extensive double bond migration and racemization occur with supported and unsupported palladium catalysts. Most, if not all, of the racemization results from double bond migration. Racemization of the alkenes by other processes was not detected. The rate of double bond migration is reduced markedly by the presence of base.”
What is the difference between pd H2 and Pd/c H2
Probably someone forgot to write /C in the first case.
I’m doing a research related to Pd/C catalyst used in hydrogenation process. My objective is how to reduce the loss of catalyst after 20 cycle of hydrogenation process.
During filtration after the process of hydrogenation there will be any loss of Pd\C can occur or not?
The reaction is usually filtered over Celite and that is how the solid Pd/C is removed from the reaction mixture.
Where can I find more info on Pd vs Pt reactivity differences? Is one (hydroxide) more likely to cause dechlorination than the other?
Hi – Pd(OH)2 (Pearlman’s catalyst) is generally pretty active. To directly compare Pd vs Pt hydrogenation catalysts I would suggest going to Encyclopedia of Reagent for Organic Synthesis (the electronic version). If you don’t have that, then there is a version available on the Internet Archive you can view for free:
https://archive.org/details/encyclopediaofre0006unse_i0i6
whether presence of chloride has impact on hydrogenation reaction using Pd/C in acetic acid medium.
Do you mean a C-Cl bond in the molecule you are planning to hydrogenate? Or if you are adding NaCl or some other chloride salt?
James if I have H2(gas, 1 atmosphere) in pd/carbon,ethanol whether it can reduce double bond or carbonyl carbon to alcohol and sir pls tell me reason why it cannot reduce carbonyl compound and if I want to reduce carbonyl compound what temperature and pressure I should have ?????
I would look for a literature precedent on the reduction you are trying to achieve. Typically C=O bonds require more heat and pressure to reduce than olefins.
How to reduce the double bond without disturbing the benzyl group or PMB?
Generally hydrogenation of alkenes is easier than reducing off a benzyl group. On a practical basis hydrogenating an alkene is usually done under 1 atm atmosphere of H2. If you want to remove a benzyl group it generally requires slightly higher pressure (e.g. Parr reactor at 4 atm) or higher catalyst loading. If it’s very finicky you can try doing a hydrogenation with diimide which is very mild.
What would be the product of an acid chloride with H2/Pd-C
Likely an aldehyde. If the palladium catalyst has a bit of barium sulfate present the reduction will stop at the aldehyde stage and not go further to the alcohol. https://en.wikipedia.org/wiki/Rosenmund_reduction
Sir can this also reduce ketones, acid and alpha-substituted aldehydes ?
It can, but reduction of ketones requires significantly higher pressures.
and I have amide group and ester group, i just want to reduce ester group
Lithium borohydride should do the trick.
how to prepare Pd/C (10%w) in reaction?
See the references.
Does H2 Pd reduce carbonyl groups ?
Given enough pressure of H2 and high enough temperature, absolutely.
By what mechanism is the H2 attacking the pi bond? And why don’t carbonyl grp don’t get reduced at normal condition even if the c=o bond the polar in nature?
Well that is a very good question, and generally doesn’t get covered in an intro course. What happens is that the Pd forms a transitory bond with the sigma bond in the H-H molecule, and then, by a mechanism known as “oxidative addition” that H-H bond is broken and two new Pd-H bonds are formed. Then, Pd bonds to the alkene, and through another mechanism we don’t cover called, “migratory insertion” one of the new C-H bonds is formed, followed by a last mechanism called, “reductive elimination”. This forms the second C-H and brings the oxidation state of the Pd back to 0 so it can re-enter another catalytic cycle.
So there’s a lot going on at the surface of the Pd that we don’t really cover here because it’s best handled in introductory inorganic chemistry classes.
The question about reducing C=O is a longer answer – it has to do with the fact that Pd is a late metal and tends to bind better to alkenes than highly polar carbonyls.
For all of the examples given with an aromatic ring, will those reactions only if the substituent being reduced is on benzene? For example, will a regular imine also be reduced?
With enough pressure, yes. Imine reduction is usually done with NaCNBH3 or LiAlH4. The one reaction where Pd-C/H2 works best next to an aromatic ring is reduction of a ketone to an alkane. Very difficult to do with a “normal” ketone but it can be done when the ketone is adjacent to an aromatic ring.
Sir H2/pd/c can reduce esters to alcohol?
Given enough heat and pressure, certainly.
What happens if a reactant contains both a double and a triple bond and is reduced with H2/Pd?
Hard to get selectivity for alkene over alkyne with H2/Pd. You’ll likely get complete reduction to the alkane, containing no double bonds. If you want selective reduction of the alkyne to the alkene, use Lindlar’s catalyst.
Hahaha… The matchmaker example was excellent.. That just brought the laugh.. Enjoyed reading.. Understood well.. Thanks!!!
how can calculate equivalent of Pd/C for reduction of nitro is there any specific equivalent?
Does it reduces esters and carboxylic acid,
Not the best choice. Would require tremendously high pressures. Use LiAlH4.
Hey there! I really appreciate this website, it’s been a lifesaver since I started Organic Chemistry! I have a question about Pd/C H2 — does it reduce carbonyls as well as alkenes/alkynes?
It *can*, although generally higher pressures of H2 are required for reducing carbonyls.
“palladium (Pd) or platinum (Pt) are all equivalent to each other.” , “There are subtle differences in reactivity.”
Sir , could you list out the differences?
One really subtle effect is that stereocenters adjacent to a pi bond can get epimerized. Saw this one in a dihydrofuran system. Epimerization was highest in Pd(OH)2 (1:1), lower with Rh/Al2O3 (7.5:1), and even lower with PtO2 (20:1). No scrampling with HN=NH.
Some cationic hydrogenation catalysts can be directed with pendant hydroxyl groups.