Aldehydes and Ketones
Imines – Properties, Formation, Reactions, and Mechanisms
Last updated: February 19th, 2025 |
Imines – Their Properties, Formation, Reactions, and Mechanisms
- Imines are the nitrogen analogues of aldehydes and ketones, containing a C=N bond instead of a C=O bond.
- They are formed through the addition of a primary amine to an aldehyde or ketone, kicking out a molecule of water (H2O) in the process.
- In this post we show some examples of the synthesis of imines, walk through the mechanism for their formation, provide a few of their reactions and compare their properties to those of aldehydes and ketones.
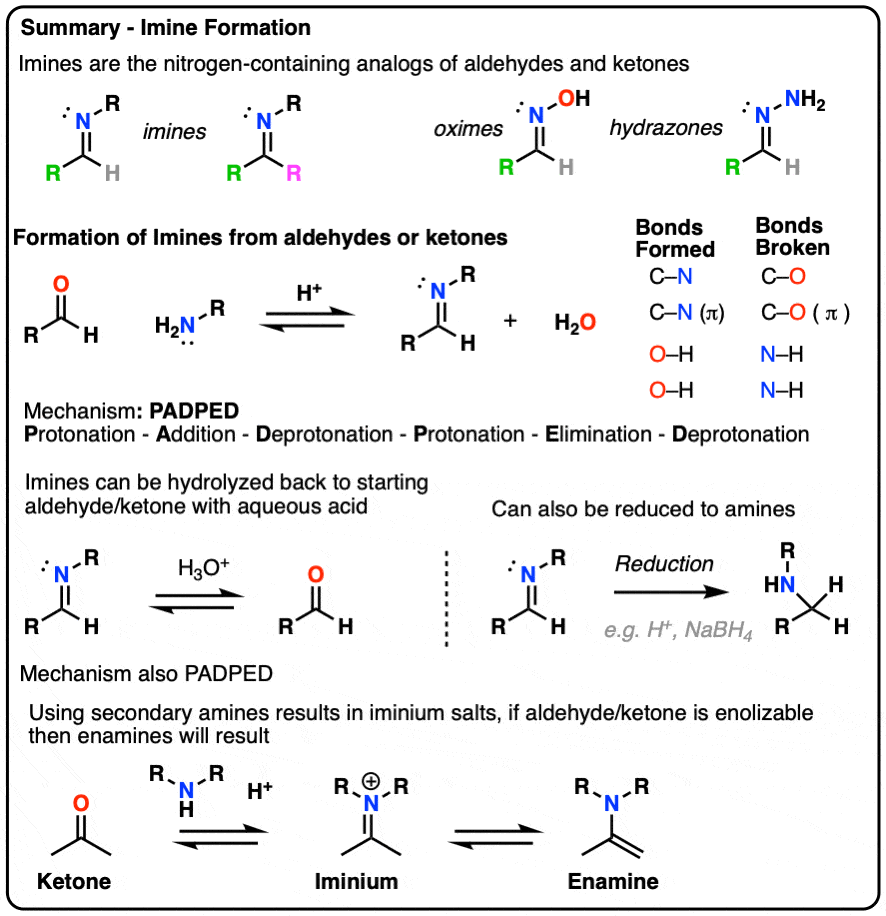
Table of Contents
-
- Imines – The Nitrogen-Containing “Cousins” Of Aldehydes and Ketones
- Synthesis of Imines From Aldehydes and Ketones
- Mechanism For The Formation of Imines – ADPED (Addition-Deprotonation-Protonation-Elimination-Deprotonation)
- Intramolecular Imine Formation
- Reactions of Imines – Hydrolysis
- Properties of Imines vs. Aldehydes/Ketones
- Cousins of Imines: Oximes and Hydrazones
- Why Can’t We Use Secondary Amines To Make Imines? (Iminium Salts and Enamines)
- Conclusion
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Imines
Today we look at aldehydes and ketones and ask: what if we swap out the oxygen for nitrogen?
That would give us an “imine“: [Note 1 – you may also see the term “Schiff base” used as well]
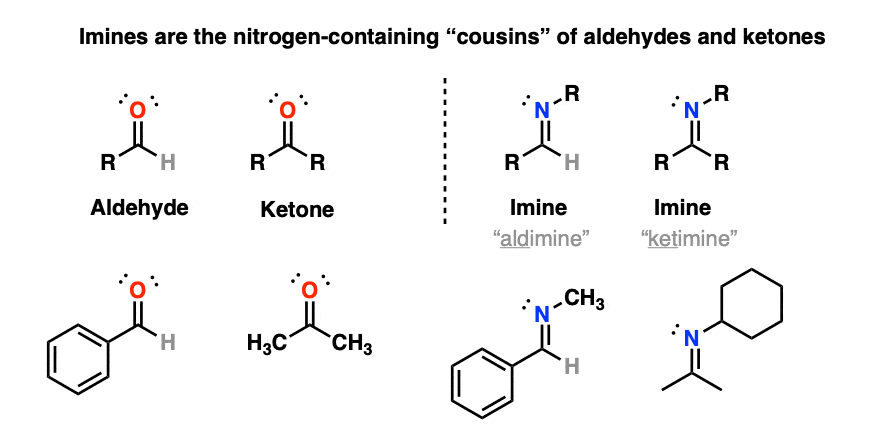
(Occasionally you may see the term “aldimine” used to refer to an imine made from an aldehyde and “ketimine” for an imine made from a ketone, but we’ll be using the general term “imine” to refer to both here. )
2. Synthesis of Imines From Aldehydes and Ketones
The synthesis of imines is simple enough in most cases. A primary amine is added to a solution an aldehyde or ketone, water (H2O) is liberated and the corresponding imine is formed. You may recall that a reaction that forms water can be referred to as a condensation reaction. This is a classic example.
Here’s a specific example of imine formation from benzaldehyde and ethylamine, from Organic Syntheses Coll. Vol. 5, 736 (open-access, click to see procedure).
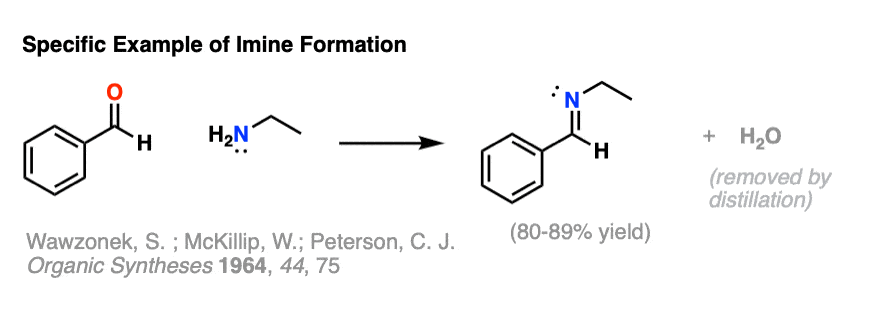
In this case, condensation is extremely rapid. The water that is formed is subsequently removed through distillation to give a crystalline imine product in high yield.
Although not absolutely required in most cases, the reaction is sped up considerably with the addition of an acid catalyst, and going forward we will assume that acid is being used. [Note 2]
If you’ll recall, there are two main pathways by which acid catalysis can help to accelerate reactions.
- Acid speeds up elimination reactions, by converting potential leaving groups into their conjugate acids and thereby making them into better leaving groups. [“The conjugate acid is always a better leaving group“]
- Acid speeds up addition reactions, by protonating the carbonyl oxygen, thereby making the carbonyl carbon a better electrophile.
Here’s a general example of imine formation, showing the bonds that form and break.
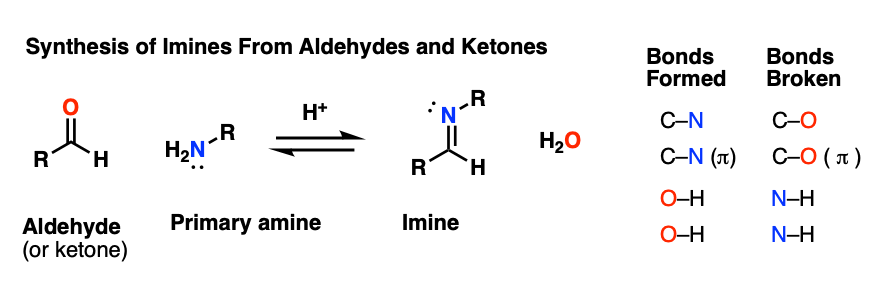
As you can readily see,
- two new C-N bonds and two O-H bonds are formed, and
- two C-O bonds and two N-H bonds are broken.
One other thing to note. The starting materials (aldehyde/ketone and primary amine) and the products (imine and water) are in equilibrium.
How might we drive the reaction forward towards the desired imine product?
As you might predict from applying Le Chatelier’s principle, it would help to remove water as the imine is formed so as to prevent the reverse reaction from happening. (In practice, this is often done by using a drying agent such as MgSO4 or molecular sieves, or by sequestering the water with a Dean-Stark Trap. )
3. Mechanism
This brings us to the next big question. How does this reaction work?
What mechanistic steps might happen en route to formation of an imine from an aldehyde or ketone?
Before I “spoil” it by showing what’s been experimentally determined, it might be a fun exercise to make a prediction. So I encourage you to take a stab for yourself. Some things to keep in mind:
- Any proposed mechanism for this reaction has to account for the breaking of two C-O bonds and two N-H bonds as well as the formation of two C-N bonds and O-H bonds.
- Recall that the carbonyl carbon of aldehydes and ketones is an excellent electrophile and can undergo addition reactions with good nucleophiles. (Hint: amines are nucleophiles).
- For our purposes, assume the reaction uses an acid catalyst. Recall that acid makes C=O bonds into better electrophiles, and that the conjugate acid of any base is a better leaving group.
What do you think happens?
Let’s dive in.
In the presence of mild acid, there will be an equilibrium between the neutral aldehyde/ketone and the conjugate acid of the aldehyde/ketone, so step 1 is shown as protonation of the oxygen (form O-H). [Note 3 ]
Once protonated, the carbonyl can undergo addition of the amine nucleophile to the electrophilic carbonyl carbon, forming C-N and breaking C-O (pi).
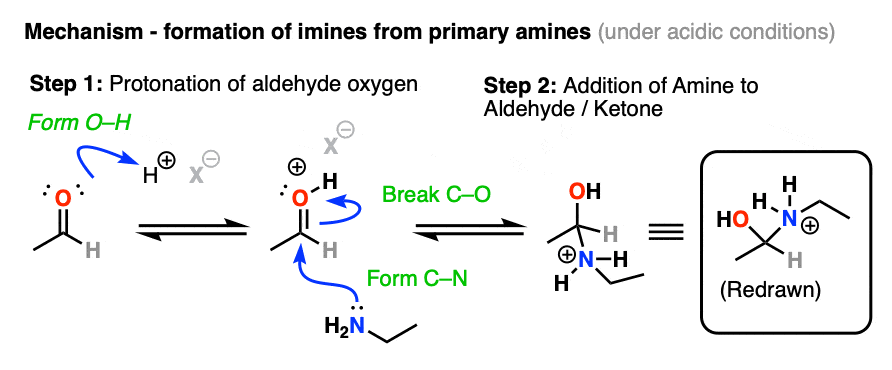
The hybridization of the carbon has changed from sp2 to sp3. We now have a tetrahedral intermediate with a neutral OH and a positively charged amine (i.e. an ammonium). [Note 4]
So what comes next?
- We still have to form O-H and C-N (pi), and we still have to break C-O and two N-H bonds.
- Elimination is unlikely to occur at this point, because the nitrogen has no available lone pair with which to form C-N (pi), and leaving group would be the hydroxide ion, HO(-), a relatively strong base.
It would be really nice to be able to shift a proton from nitrogen (break N-H) to oxygen (O-H)!
Deprotonation (Step 3, break N-H) of the nitrogen by a base (e.g. excess amine, although it’s fine to just write B: since many other species could do this) followed by protonation (Step 4, form O-H) of oxygen by acid achieves this.
Watch out – it is generally a mistake to show this happening in one step by using the OH to deprotonate N-H. Showing a two-step process is more correct.
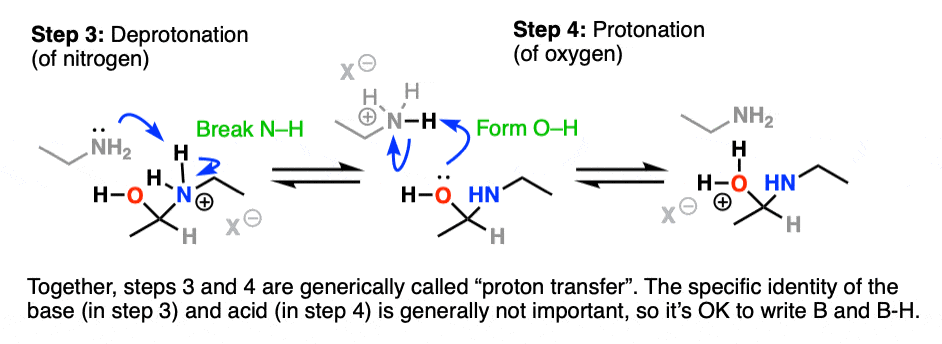
Together these two steps effect the shifting of a proton (H+) from nitrogen to oxygen. For this reason the process of deprotonation-protonation (or its reverse) are commonly referred to as proton transfer.
Protonation of OH to give OH2(+) has resulted in a much better leaving group.
Our checklist of bonds to form and break still requires formation of C-N (pi) and breakage of C-O. This can now be be accomplished through elimination of water with accompanying formation of C-N (pi) (Step 5, form C-N, break C-O)/
In the absence of acid, elimination is the rate-determining step in the formation of most imines; addition of acid greatly accelerates the rate of this step. [Note 5]
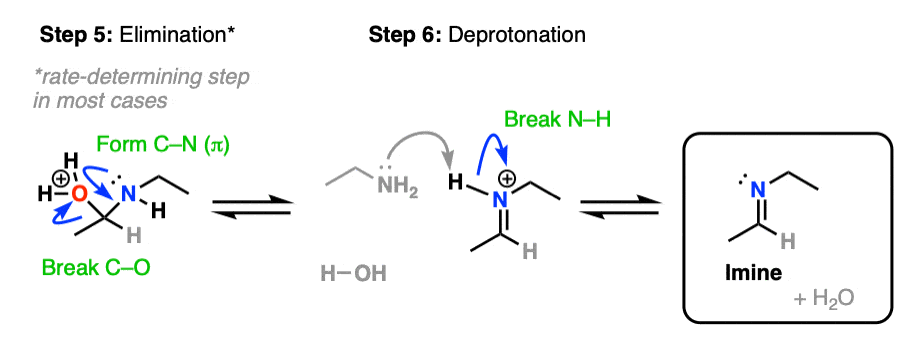
Another note – if acid is present, it’s important to show the leaving group as H2O, not HO- , since HO- is a strong base and won’t exist in the presence of strong acid.
Elimination results in the conjugate acid of the imine, referred to as an iminium salt (or iminium ion). Deprotonation of the positively charged nitrogen (Step 6, break N-H) gives us the neutral imine.
Putting these steps together we have Protonation- Addition – Deprotonation – Protonation – Elimination – Deprotonation, or PADPED. (Remember this mnemonic, because it comes up a lot!)
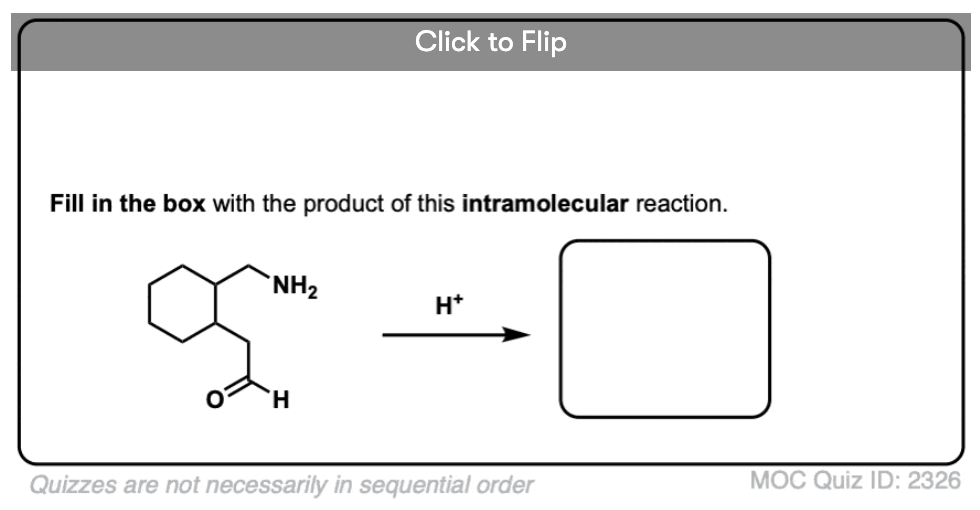
4. Intramolecular Imine Formation
It is perfectly possible for an amine and an aldehyde/ketone to be present on the same molecule, attached through a carbon chain. Formation of a cyclic imine is particularly likely if a five- or six-membered ring can be formed.
It’s the exact same sequence of steps as in the intermolecular case, it just so happens that the amine and aldehyde are connected through a carbon chain; here’s an example.
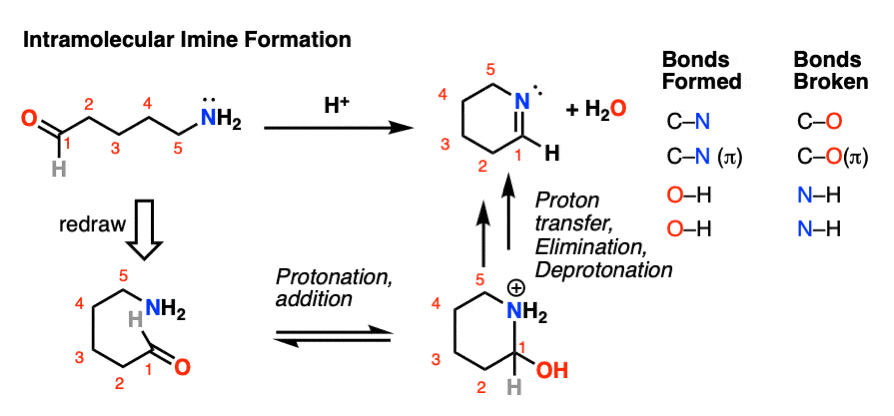
For mechanism, hover here or click on this link.
5. Reactions of Imines
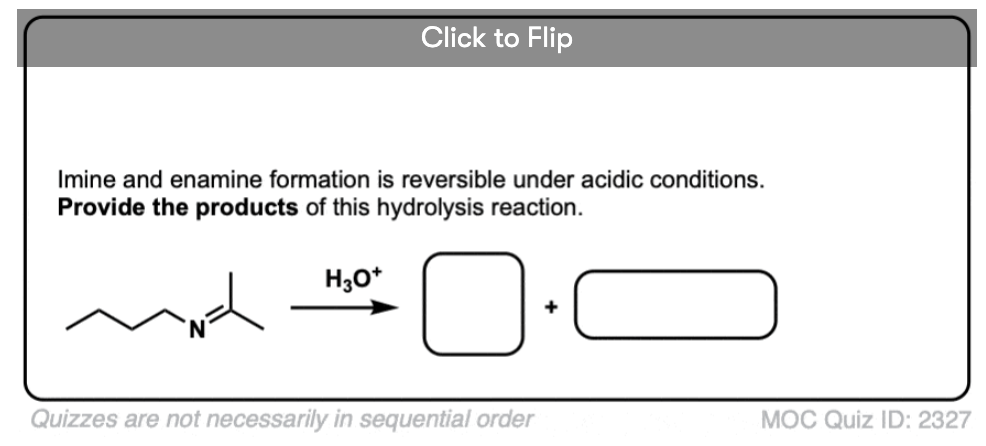
For our purposes, probably the most important reaction of imines is hydrolysis, where treatment of the imine with water (usually aqueous acid) restores the original aldehyde/ketone and primary amine.
As you might expect for the reverse of imine formation, two C-O bonds (and two N-H bonds) are formed, and two C-N bonds (and two O-H bonds) are broken.
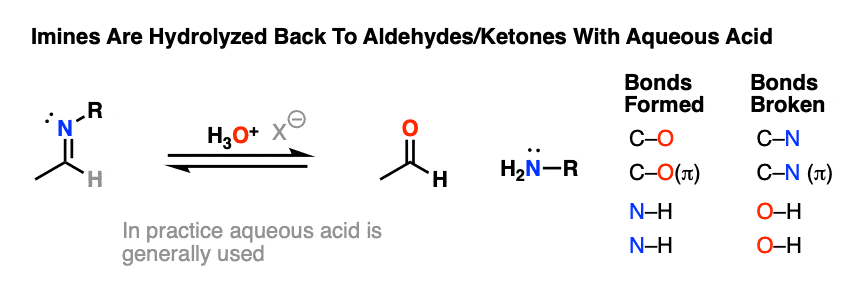
Aqueous acid (H3O+) is commonly used to drive the equilibrium towards hydrolysis. Under these conditions, the mechanism for hydrolysis using aqueous acid is Protonation – Addition – Deprotonation – Protonation – Elimination – Deprotonation (PADPED. )
That’s not a typo!
The hydrolysis of imines has the exact same sequence of steps as the formation of imines, except that the identities of the nucleophile, electrophile, and leaving group are all switched around.
Hover here for mechanism or click on this link.
Imines are capable of undergoing addition reactions just as aldehydes and ketones are.
One important reaction of imines is reduction with a source of hydride (H-) to give amines. Of particular importance is a process known as reductive amination whereby an aldehyde or ketone is combined with a primary amine and a reducing agent resulting in the direct formation of amines. [Note 6]
6. Properties of Imines
Having gone through the reactions by which imines are formed (and destroyed), now we get to the fun part. Thinking through periodic trends!
How do you think the properties of imines might differ from aldehydes/ketones? What might be the effect of removing the oxygen and replacing it with the atom immediately to its left on the periodic table?
Let’s start with what might be an easy question. What functional group has a higher dipole moment – an imine or a ketone?
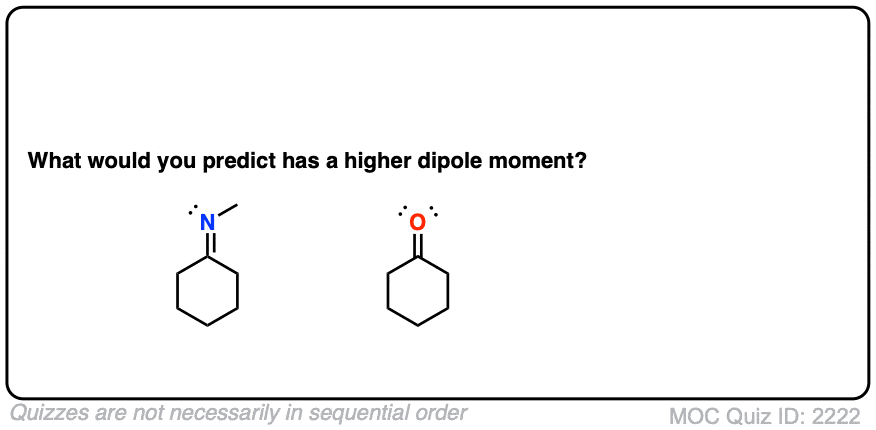 Click to Flip
Click to Flip
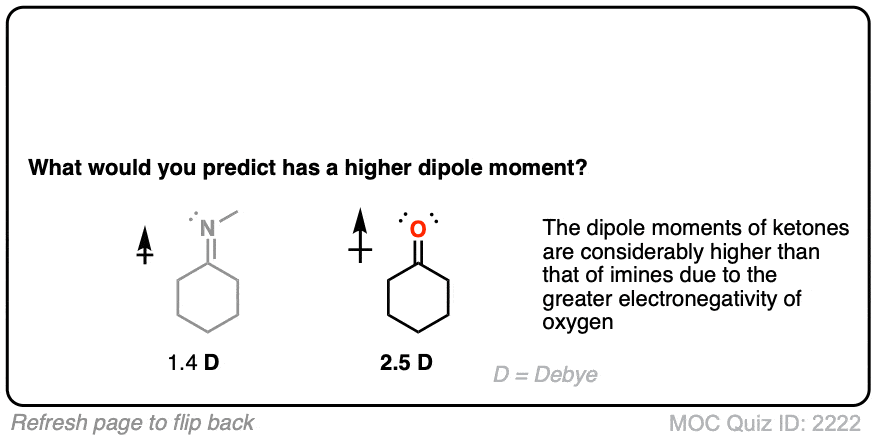
Given the higher electronegativity of oxygen, this shouldn’t be too surprising.
Let’s think along a different direction. What do you think might be more basic: an aldehyde or an imine?
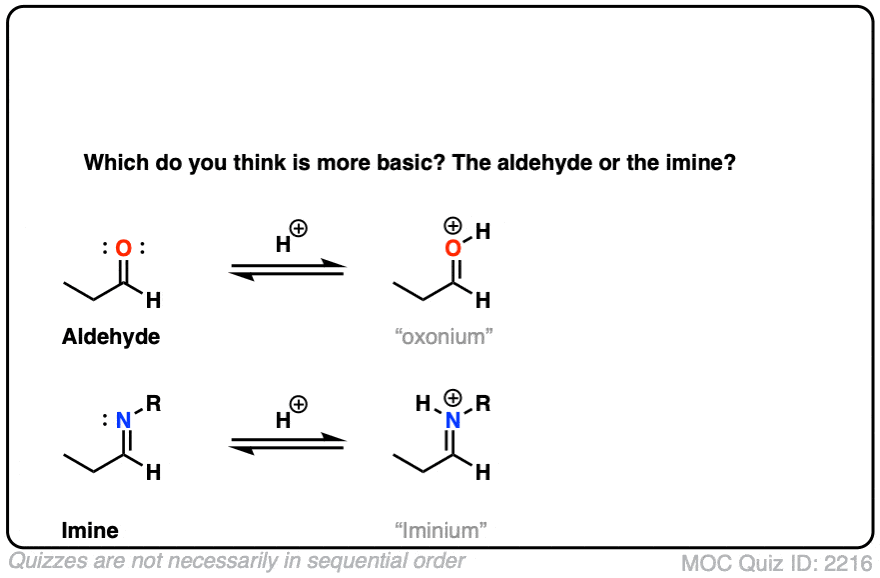 Click to Flip
Click to Flip
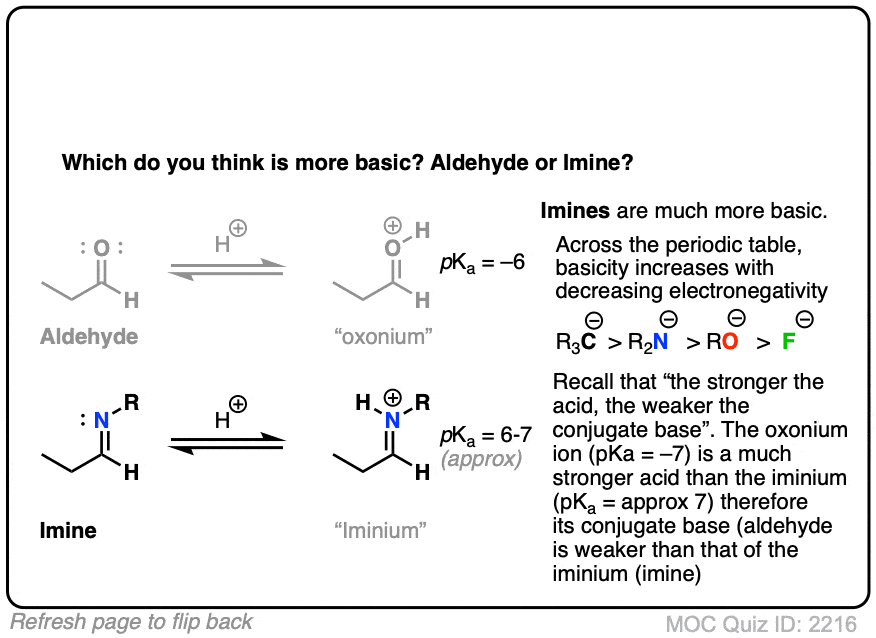
Recall that basicity increases as we move to the left on the periodic table; the electrons are less tightly held (electronegativity decreases) and therefore more easily donated to electrophiles.
Imines are considerably more basic. The pKa of a typical iminium (protonated imine) is around 4 or 5. The pKa of a protonated aldehyde is about –7 [Ref].
Since “the stronger the acid, the weaker the conjugate base”, this makes imines about 11 or 12 pKa units (= 12 orders of magnitude, 1012 ) more basic!
Third question.
What do you think is more electrophilic (i.e. more reactive towards nucleophiles): aldehydes or imines?
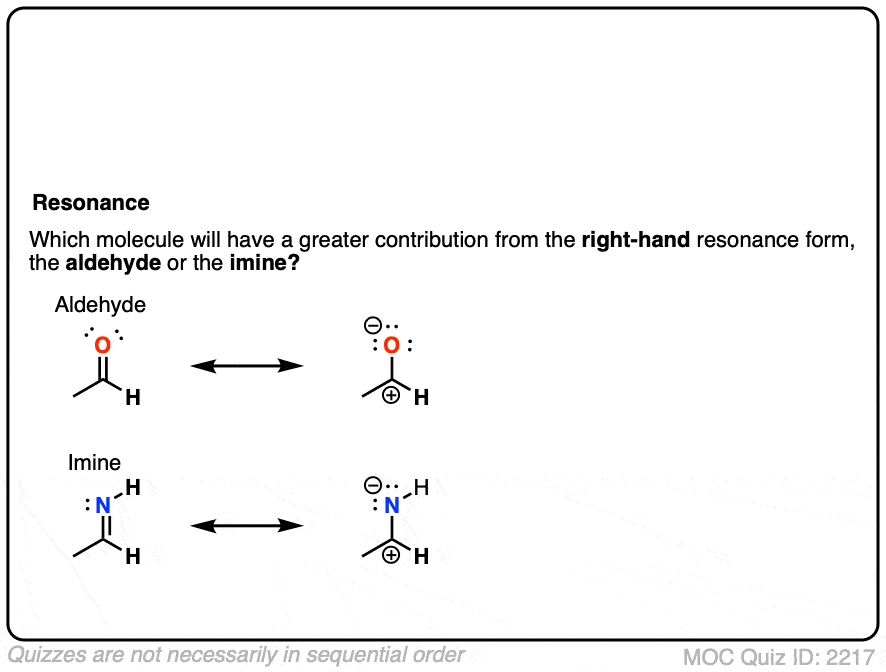 Click to Flip
Click to Flip
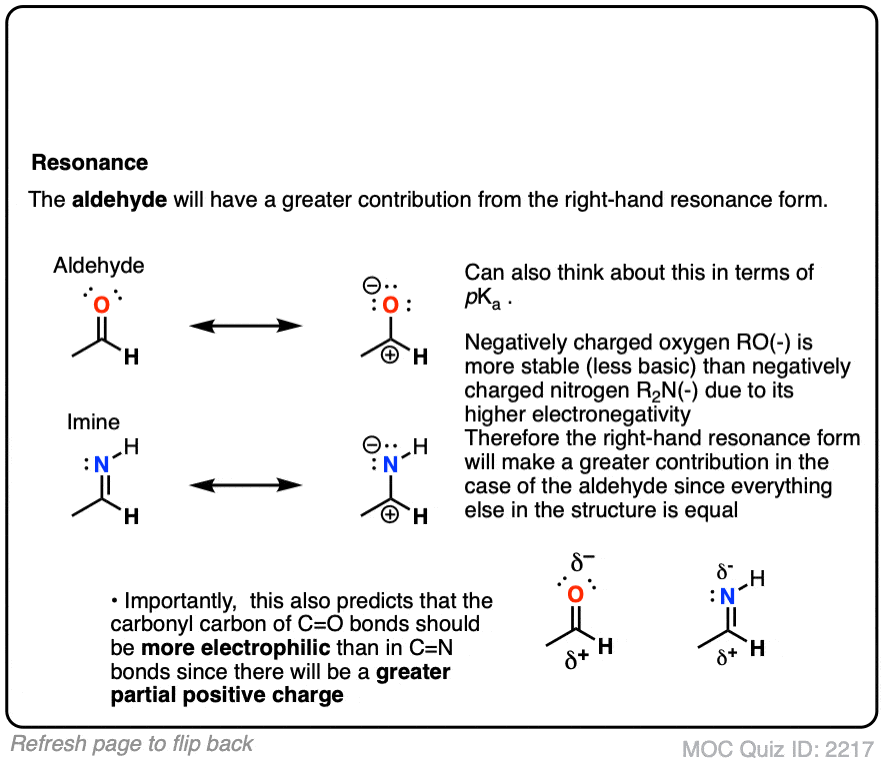
All else being equal we should expect aldehydes/ketones to be more electrophilic than their imine counterparts.
- Oxygen is more electronegative than nitrogen, so the greater dipole moment should result in a greater partial positive charge on carbon, making it more electrophilic.
- Same argument, but backwards: N is a better electron donor than O, which will make the attached carbon more electron-rich and therefore less electrophilic.
- It’s also worth thinking about “second-best” resonance forms for both the aldehyde and imine, which so often dictate their reactivity. It can be helpful to think of “higher basicity” as being a proxy for “more unstable” and therefore “less likely to contribute to a resonance hybrid”. With the aldehyde, we have O(-) (the conjugate base of an alcohol, pKa about 16 ) on the right, whereas with the imine, RN(-) is on the right (the conjugate base of an amine, pKa about 38).
Here is a bonus question, which I will leave for the footnotes: What do you think is more electrophilic (i.e. reactive towards nucleophiles) – an imine, or a protonated imine (“iminium”). [Note 8]
7. Cousins of Imines: Oximes and Hydrazones
While on the topic of imines, it’s worth noting that these functional groups have “cousins” that make cameo appearances in introductory courses and therefore shouldn’t be left out.
Oximes and hydrazones are formed from aldehydes/ketones through a similar mechanism to that for the formation of imines.
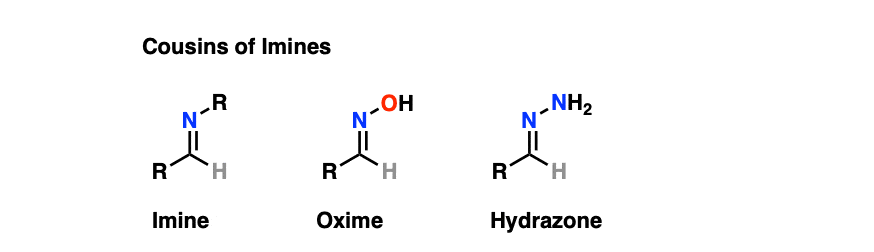
- Oximes are formed through treatment of aldehydes or ketones with hydroxylamine (NH2OH) whereas hydrazones are formed through treatment of aldehydes/ketones with hydrazine NH2NH2. As with other imines, acid catalysis speeds up the reaction (particularly the elimination step).
- Oximes are the substrates in the Beckmann rearrangement, very important in the production of nylon [Note 9 ]
- Hydrazones are used in the Wolff-Kishner reaction that reduces a C=O bond to CH2.
The mechanism for formation of oximes and hydrazones is exactly the same as that for the formation of imines.
If acid is present, the mechanism is Protonation- Addition-Deprotonation-Protonation-Elimination-Deprotonation (PADPED).
Hover here for the oxime mechanism. or click on this link.
Hover here for the hydrazone mechanism or click on this link.
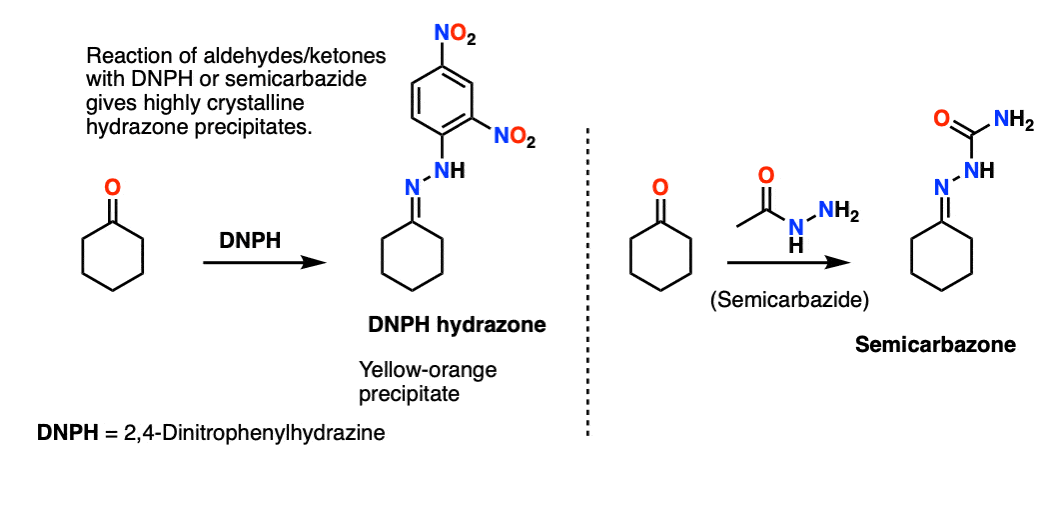
These are often encountered in laboratory courses. The DNPH (2,4-dinitrophenylhydrazine) and semicarbazide derivatives of aldehydes and ketones often provide highly crystalline, brightly colored hydrazone precipitates with sharp melting points. [Note 10]
8. Why Can’t Secondary Amines Be Used To Make Imines?
At the top we mentioned that imines are made through the treatment of an aldehyde or ketone with a primary amine.
Why just primary amines? Why can’t we use a secondary amine instead?
Treatment of an aldehyde or ketone with a secondary amine results in iminium salt , which may or may not (depending on its structure, see below) undergo further reaction to yield an enamine.
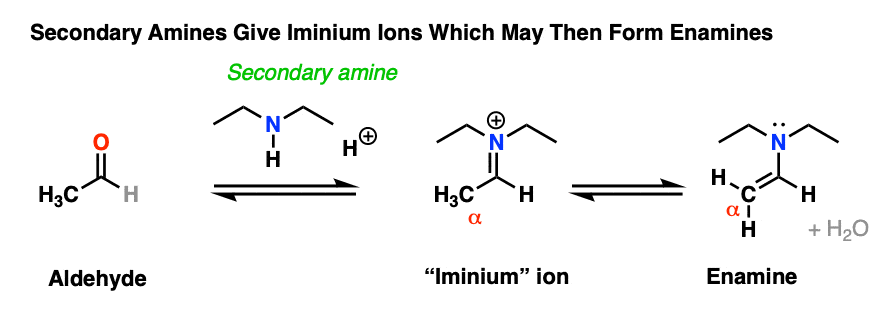
Why?
It’s worth walking through in detail, because the addition of secondary amines resembles that of primary amines – up until the very end.
Let’s go through the mechanism.
Assuming acid is present, the first step would be protonation of the carbonyl oxygen (forming O–H) followed by addition of the amine lone pair to the carbonyl carbon, (form C-N, break C-O (pi) ).
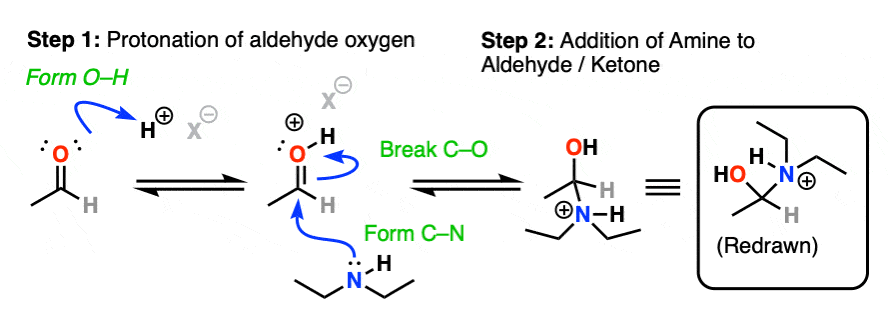
Next comes our proton transfer step. Deprotonation of N (break N-H) followed by Protonation of O (form O-H) gives us the excellent leaving group R-OH2(+)
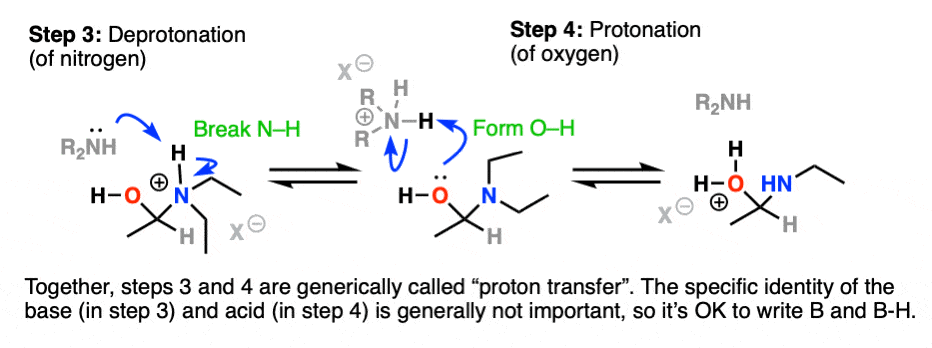
With water as a leaving group, elimination is now much more likely to occur, resulting in formation of C-N (pi) and breakage of C-O.
This gives us this positively charged iminium salt, below.
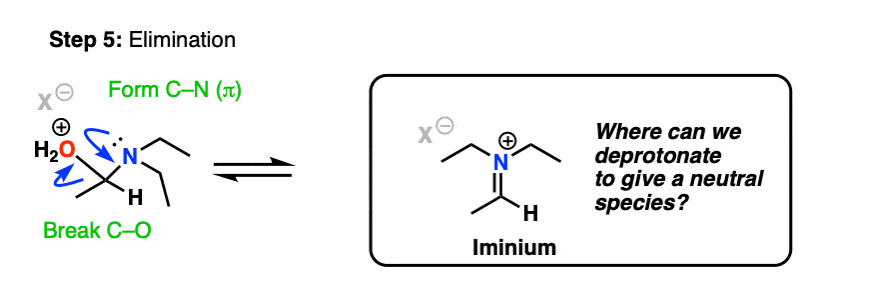
If we’re following PADPED, he next step should be Deprotonation, right? Here’s the problem.
The nitrogen is already bound to three carbons. There’s no proton to pull off from the nitrogen!
In the case of a molecule like formaldehyde (and other “non-enolizable” aldehydes/ketones that lack a proton on the alpha carbon), this is where things stop. We obtain an iminium salt as our final product. [Note 11]
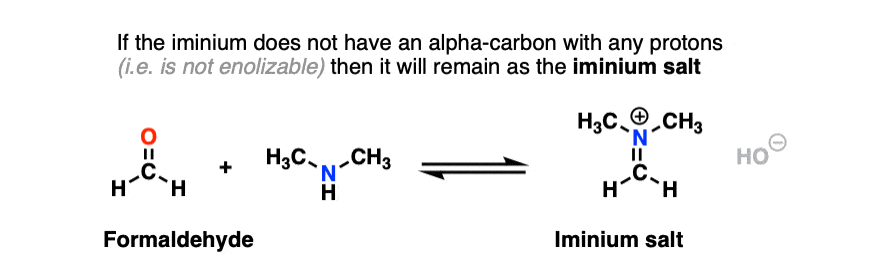
However, if there is a proton on the alpha carbon of the iminium (i.e. it is enolizable), we can remove a proton from that position.
Deprotonation (break C-H, form C-C (pi), break C-N (pi) gives us the following species:
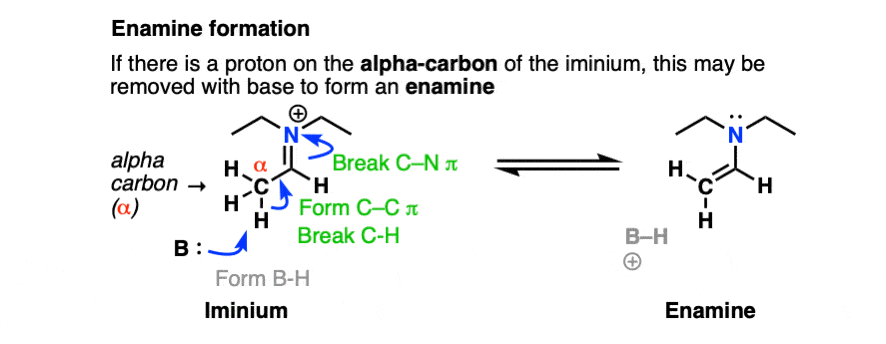
Note that it has an alkene attached to an amine. So, like the combination of spoon and fork gives us a spork, custom dictates that this go by the name, enamine (a name first coined by Nobel laureate George Wittig back in 1927).
If you’re keeping score, this is still P A D P E D for enamine formation. However, this time, the Deprotonation happens on the alpha carbon.
Enamines are excellent nucleophiles and undergo a completely different set of reactions. It’s probably best to stop at this point and deal with them in a separate article.
Just to give you a taste, here is an example of enamine formation from Organic Syntheses.
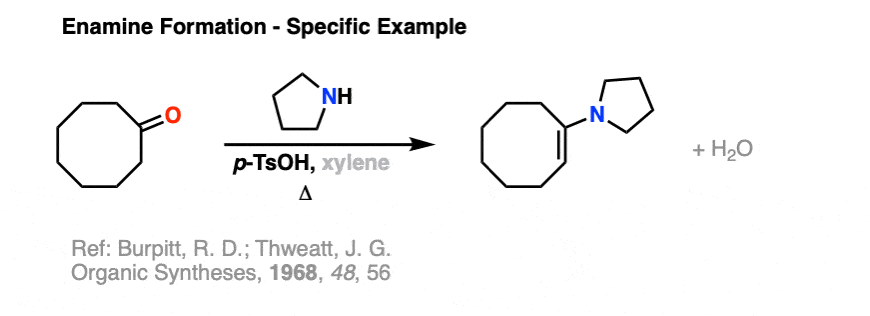
Reference: Organic Syntheses, 1968, 48, 556.
9. Conclusion
So what have we learned about imines?
- Imines are the nitrogen-containing cousins of aldehydes and ketones
- They are formed through treatment of an aldehyde or ketone with a primary amine. Acid helps to catalyze the reaction.
- The mechanism for formation under slightly acidic conditions is Protonation-Addition-Deprotonation-Protonation-Elimination-Deprotonation, or P A D P E D.
- If an aldehyde/ketone and primary amine are on the same molecule, intramolecular imine formation may result, giving a cyclic imine.
- Imines can be hydrolyzed back to the parent aldehyde/ketone with H2O (in practice, usually aqueous acid H3O+), again through P A D P E D.
- Imines may also be reduced with a hydride source (H–) to give amines. Combining imine formation with reduction is called reductive amination.
- Imines are more basic than aldehydes/ketones, and generally are less reactive towards nucleophiles.
- Oximes and hydrazones are chemical cousins of imines that are formed via the same mechanism.
- Treatment of aldehydes/ketones with secondary amines results in iminium salts, which may be further deprotonated (e.g. at the alpha carbon) to give enamines. The mechanism for formation of enamines is also P A D P E D.
Here is a potentially useful figure summarizing the mechanisms of the forward and reverse reactions:
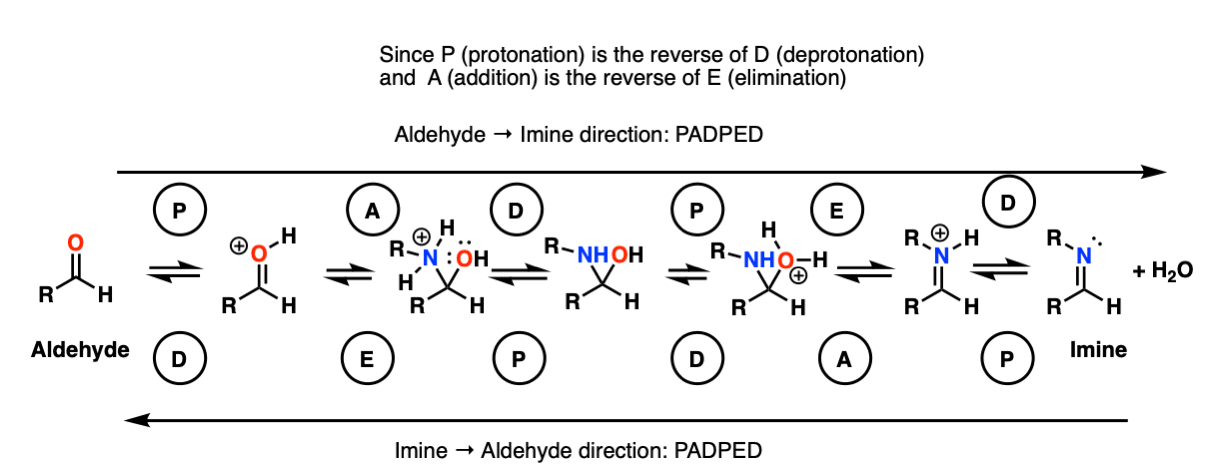
We will dive into the chemistry of enamines in the next article in this series, here.
Notes
Note 1- The term, “Schiff base” is often restricted to imines made from aromatic aldehydes (e.g. benzaldehyde) , which are considerably more resistant to hydrolysis (and thus easier to isolate) than those made from alkyl (“aliphatic”) aldehydes. A more rarely encountered term, “anil” refers to imines made through the condensation of aromatic amines (e.g. aniline) with aldehydes. Confusingly, the term “azomethine” also finds use for imines, particularly in the older literature.
Note 2 – Acid is not strictly required for imine formation, especially for alkyl amines (and other nucleophilic amines such as hydroxylamine / hydrazine) , where equilibrium constants >200 have been measured in aqueous solution [Ref] . For the purpose of this article we will be assuming mildly acidic conditions, although in other parts of the course (e.g. formation of the hydrazone in the Wolff-Kishner) you may see condensation reactions to give C=N under neutral or even basic conditions.
Note 3 – For alkyl amines, protonation of the aldehyde is not necessary in order for addition to occur and elimination of water is rate-determining. For weakly nucleophilic amines (e.g. PhNH2 and other amines with a pKaH of 3-5) addition is rate-determining and protonation of the carbonyl oxygen is necessary for addition to occur at a reasonable rate [See Anslyn & Dougherty, 2006, p. 597 for further discussion]. We trust that most introductory courses will not make a strong distinction between these two cases, so “protonation-first” is shown.
Note 4 – By analogy to “hemiacetal”, the neutral species that can result from the addition to an amine to an aldehyde or ketone is known as a “hemiaminal” or carbinolamine.
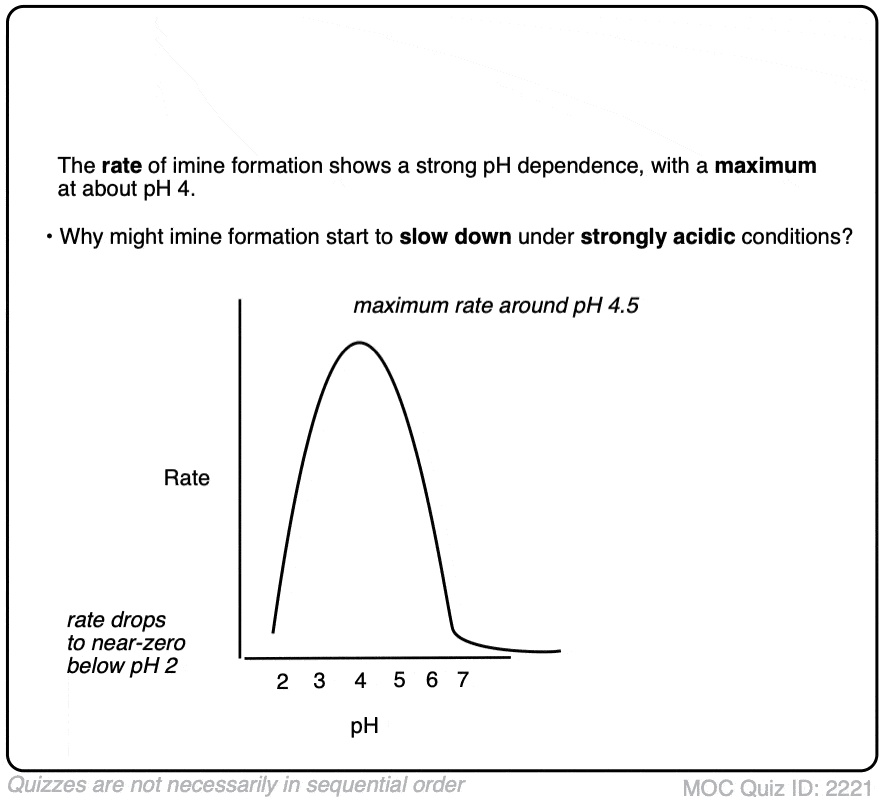
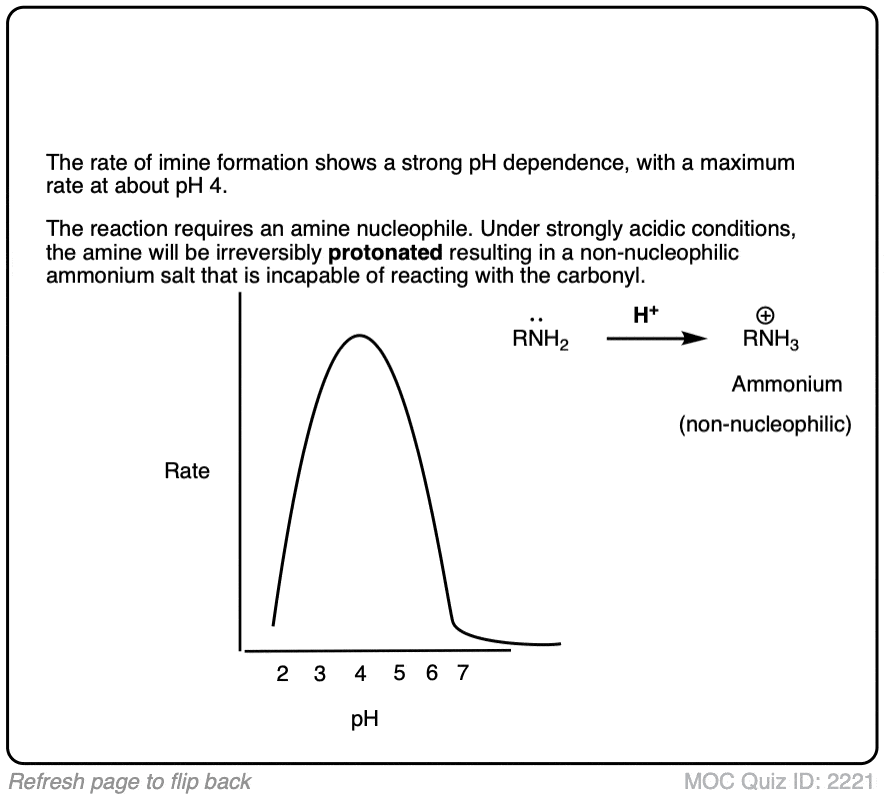
Note 5 – The formation of imines shows a strong dependence upon pH, with a typical maximum arising around pH 4-5 [e.g. Ref] . When reactions are monitored by UV, what is typically observed is a fast disappearance of the C=O absorbance (i.e. fast addition) followed by a very slow appearance of the C=N absorbance. This implies that elimination is rate determining.
What’s also interesting is that the rate starts falling as the pH decreases below 4. Why do you think that might be?
 Click to Flip
Click to Flip
Note 6 – Reductive amination is a process by which aldehydes/ketones are converted into imines and subsequently reduced without isolation of the intermediate imine. The classic method for carrying this out is by using the reducing agent sodium cyanoborohydride (NaCNBH3) at slightly acidic pH (6 or so). Under these conditions the aldehyde/ketone is reduced only slowly, whereas the iminium (the conjugate acid of the imine) undergoes smooth reduction.
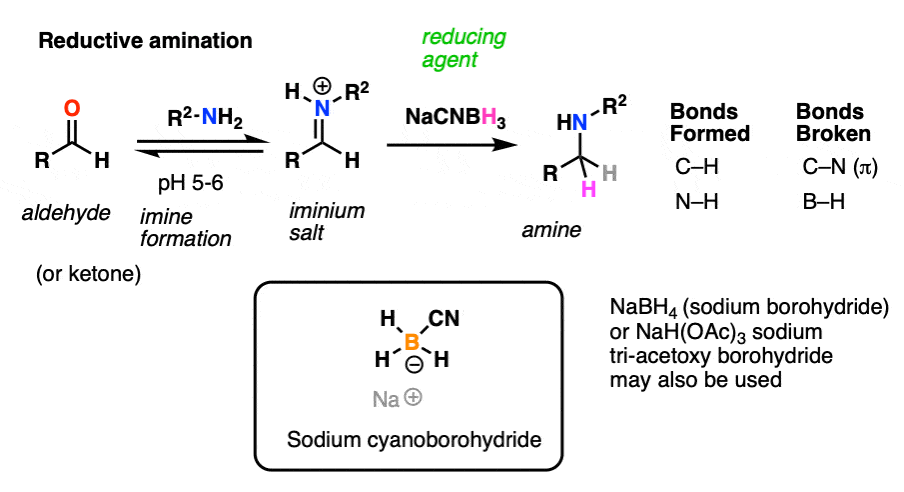
Think about that. This implies that iminium salts are more reactive towards nucleophiles than are neutral aldehydes!
Note 7 – All else being equal, aldehydes are more electrophilic than (ald)imines, and ketones are more electrophilic than (ket)imines.
Note 8 – What’s more electrophilic – an iminium (protonated imine) or an imine?
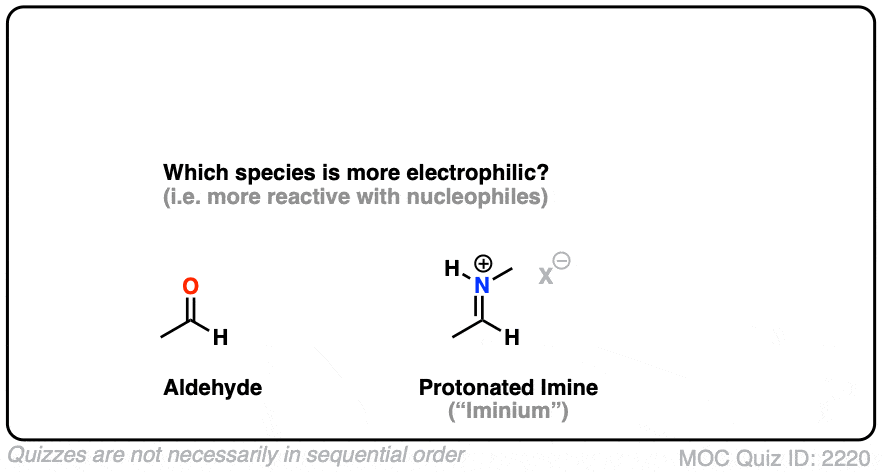 Click to Flip
Click to Flip
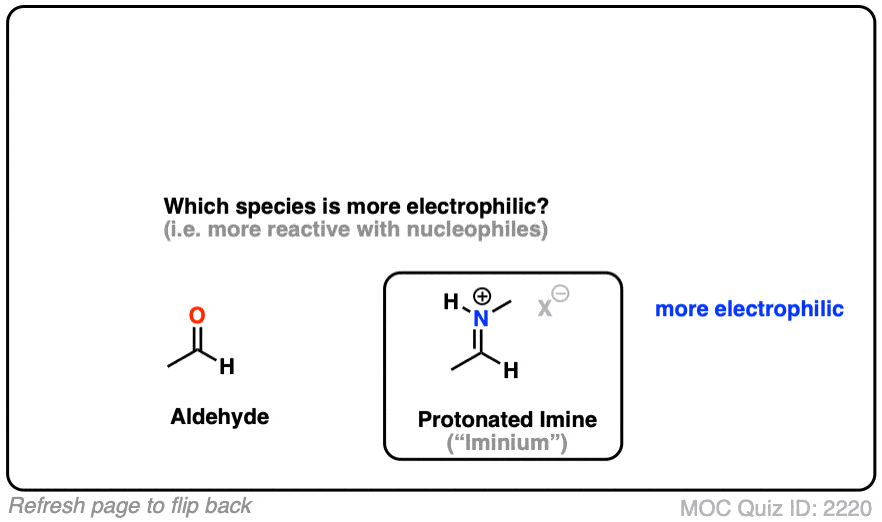
Neutral resonance forms are much more important (i.e. make a greater contribution towards the resonance hybrid) than charged resonance forms. The contribution of the imine resonance form that bears two point charges will be very low relative to that of the neutral resonance form.
Compare that to the iminium, where both of the most important resonance forms have a single point charge. While the resonance form where carbon has a full octet will be more important, the right-hand resonance form (the carbocation) will make a much larger contribution than it does in the case of the neutral imine.
Note 9 – Cyclohexanone is converted to cyclohexanone oxime, which undergoes Beckmann rearrangement upon treatment with strong acid to give caprolactam, About 5 million tons of caprolactam are made every year for the production of Nylon-6.
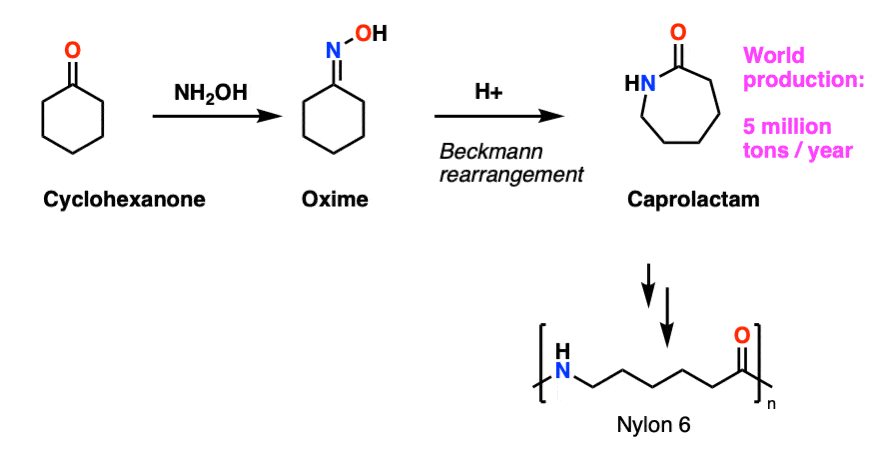
Why the name caprolactam? The six-carbon carboxylic acid CH3CH2CH2CH2CH2CO2H is called caproic acid, named for the smell of goats (old goats are particularly smelly). Esters of carboic acids are called caproates; the corresponding cyclic ester (lactone) is called caprolactone. Cyclic amides are called lactams. Caprolactam is therefore the cyclic amide derived from caproic acid.
Note 10 – The hydrazines 2,4-Dinitrophenylhydrazine (DNPH) and semicarbazide are classic reagents for testing for the presence of aldehydes/ketones in an unknown sample. This is still done in teaching labs (and in TLC stains). In the days before spectrometry, melting points were one of the few characterization tools available. Conversion of liquid ketones/aldehydes to highly crystalline DNPH or semicarbazone derivatives was a useful diagnostic tool.
Note 11 – Iminium salts can be stable enough to bottle up and sell commercially. The iminium salt below is known colloquially as Eschenmoser’s salt. It reacts as an electrophile with enols in Mannich reactions.
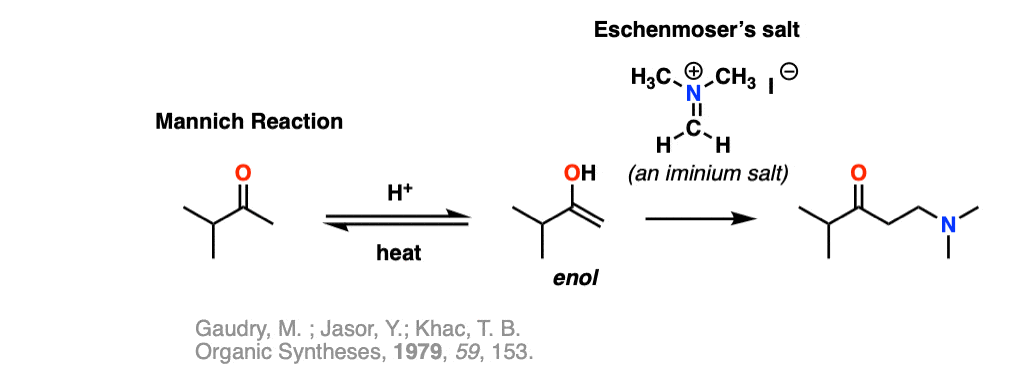
Quiz Yourself!
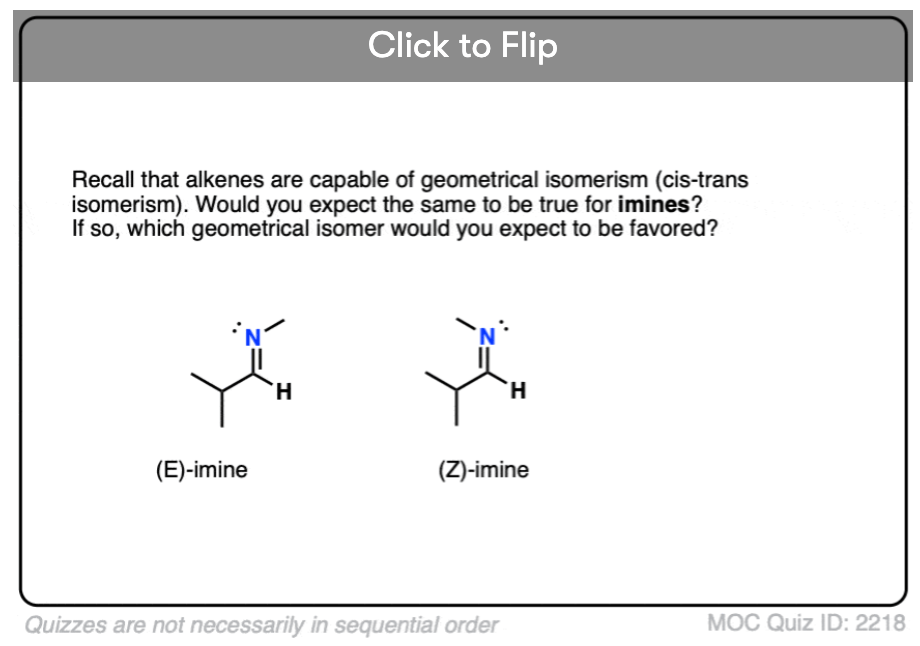
Become a MOC member to see the clickable quiz with answers on the back.
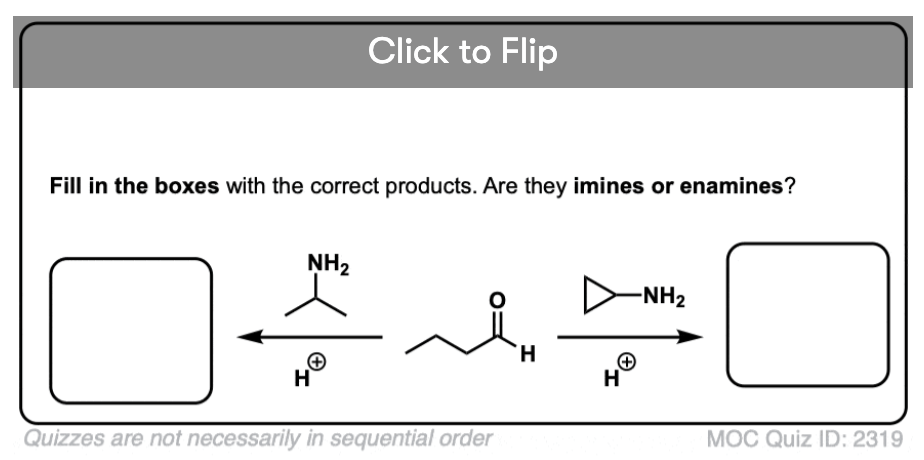
Become a MOC member to see the clickable quiz with answers on the back.
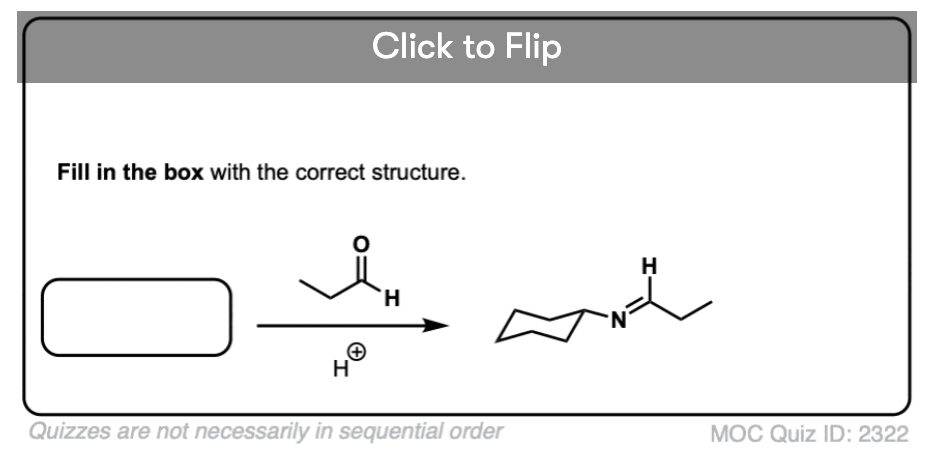
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
- The Chemistry of Imines
Layer, R. W.
Chemical Reviews, 1963, 63, 489-510
DOI: 10.1021/cr60225a003
A good overview of the key properties , reactions, and syntheses of imines, from the first isolation of an imine by Schiff in 1864 up to 1962.
Some highlights from the introductory section:
– The characteristic IR frequency for C=N ranges around 1650-1670 cm-1, contrasting with 1600-1650 cm-1in alkenes and 1710-1750 cm-1 for C=O
– Average C=N distance is about 1.24 Å for ketimines, compared to average C=O distance of about 1.21 Å for ketones
– If care is not taken, primary aliphatic aldehydes can give easily give polymeric materials with amines due to ease of aldol condensations. Trend decreases as steric hindrance of aldehyde / amine is increased.
-Aromatic aldehydes react readily and quantitatively with amines to give corresponding imines even at room temperature.
– Ketones require longer reaction times than aldehydes; acidic catalysts helpful and water removal definitely required. Aromatic ketones react even slower than aliphatic ketones with amines.
-Aldimines have lower measured dipole moments (1.4 D) than ketones (2.5 D). [Ref] -
Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen
Schiff, H. Ann. 1864, 131, 118.
DOI: dx.doi.org/10.1002/jlac.18641310113
First report of an imine, by Hugo Schiff. - PREPARATION OF KETIMINES AND THE CORRESPONDING SECONDARY AMINES
DOUGLAS G. NORTON, VERNON E. HAURY, FRANK C. DAVIS, LLOYD J. MITCHELL, and SEAVER A. BALLARD
J. Org. Chem., 1954, 19 (7), 1054-1066
DOI: 10.1021/jo01372a010
An old but useful paper that describes a straightforward synthesis of ketimines from ketones and amines, using acid catalysis and apparatus to remove water (e.g. using a Dean-Stark trap and azeotroping the water with benzene or toluene). - Studies on the Mechanism of Oxime and Semicarbazone Formation
W. P. Jencks
J. Am. Chem. Soc. 1959, 81, 475-481
DOI: 10.1021/ja01511a053
Classic study on pH dependence of oxime/semicarbazone formation (and, by implication, imines), graph on p 478 shows a maximum obtained around pH 4.5. Proposes rate-determining step is loss of water (elimination step), not protonation of carbonyl oxygen. - Synthesis of sterically hindered imines
Brian E. Love and Jianhua Ren
The Journal of Organic Chemistry 1993, 58 (20), 5556-5557
DOI: 1021/jo00072a051
A convenient synthesis of sterically hindered imines, using tetraethyl orthosilicate (Si(OEt)4) as the dehydrating agent, since it generates a nonacidic byproduct. - The Cyanohydridoborate Anion as a Selective Reducing Agent
R. F. Borch, M. D. Bernstein, and H. Dupont Durst
J. Am. Chem. Soc. 1971, 93, 2897-2904
DOI: 10.1021/ja00741a013
First detailed study on the reductive amination of aldehydes/ketones with the mild reducing agent commonly called sodium cyanoborohydide (NaCNBH3) . Shows that NaCNBH3 is a poor reductant for typical aldehydes and ketones at pH 6, but rapidly reduces imines formed from these precursors under the same conditions.
Enamine formation: - Über die Einwirkung von Ammoniak und Ammoniak‐Derivaten auf o‐Acetylaceto‐phenole
Georg Wittig, Hermann Blumenthal
Ber. 1927, 60 (5), 1085-1094
DOI: 10.1002/cber.19270600515
Nobel Laureate Prof. G. Wittig coined the term ‘enamine’ as a nitrogen analog of enol in this paper - 2,2-(TRIMETHYLENEDITHIO)CYCLOHEXANONE
R. B. Woodward, I. J. Pachter, and M. L. Scheinbaum
Org. Synth. 1974, 54, 39
DOI: 10.15227/orgsyn.054.0039
The first step in this procedure, submitted by organic chemist Prof. Robert Burns Woodward (Harvard), is an enamine synthesis. - CYCLODECANONE
D. Burpitt and J. G. Thweatt
Org. Synth. 1968, 48, 56
DOI: 10.15227/orgsyn.048.0056
The first step in this procedure from Organic Syntheses is an enamine synthesis. - Comparison of the tautomerization and hydrolysis of some secondary and tertiary enamines
Brian Capon and Zhen Ping Wu
The Journal of Organic Chemistry 1990, 55 (8), 2317-2324
DOI: 1021/jo00295a017
A study examining the stability in solution of various enamines. - Dimethyl(methyleneammonium Iodide
Angew. Chemie. Int. Ed. Engl. 10 1971 330-331
J. Schrieber, H. Maag, N. Hashimoto, A. Eschenmoser
DOI: 10.1002/anie.197103301
First report of Eschenmoser’s salt, useful electrophile for reaction with enols and enolates.
One question I would like to see answered and put in the references – energetics of imine – enamine tautomerism.
The second flipcard under “quiz yourself” has some Z products. Shouldn’t the major products be the E products?
Good catch. This needs to be fixed, thank you!