Alkyne Reactions
Partial Reduction of Alkynes With Na/NH3 To Obtain Trans Alkenes
Last updated: January 23rd, 2024 |
Partial Reduction of Alkynes to trans-Alkenes (olefins) With Sodium in Ammonia (Na/NH3)
- Alkynes can be hydrogenated to alkanes with Pd/C and excess H2, or partially reduced to cis alkenes with Lindlar’s catalyst and H2 (See article – Partial Hydrogenation of Alkynes With Lindlar’s Catalyst)
- Alkynes can also be partially reduced to trans alkenes through reduction with sodium metal (Na) in ammonia (NH3) at low temperature. This is sometimes referred to as a “dissolving metal reduction”.
- In this reaction, two electrons (from sodium metal) sequentially add to the alkyne, resulting in carbanions that are protonated by the solvent (NH3).
- The resulting trans alkenes can then undergo all the normal reactions of alkenes. It’s important to be able to properly draw the all stereoisomers resulting from the partial reduction of alkynes (with Na/NH3 or Lindlar’s catalyst) followed by stereospecific reactions such as dihydroxylation (OsO4), halogenation (Cl2, Br2), epoxidation (m-CPBA) and others.
- In the forward direction, it’s also important to be able to apply the partial reduction of alkynes toward the synthesis of various target compounds.
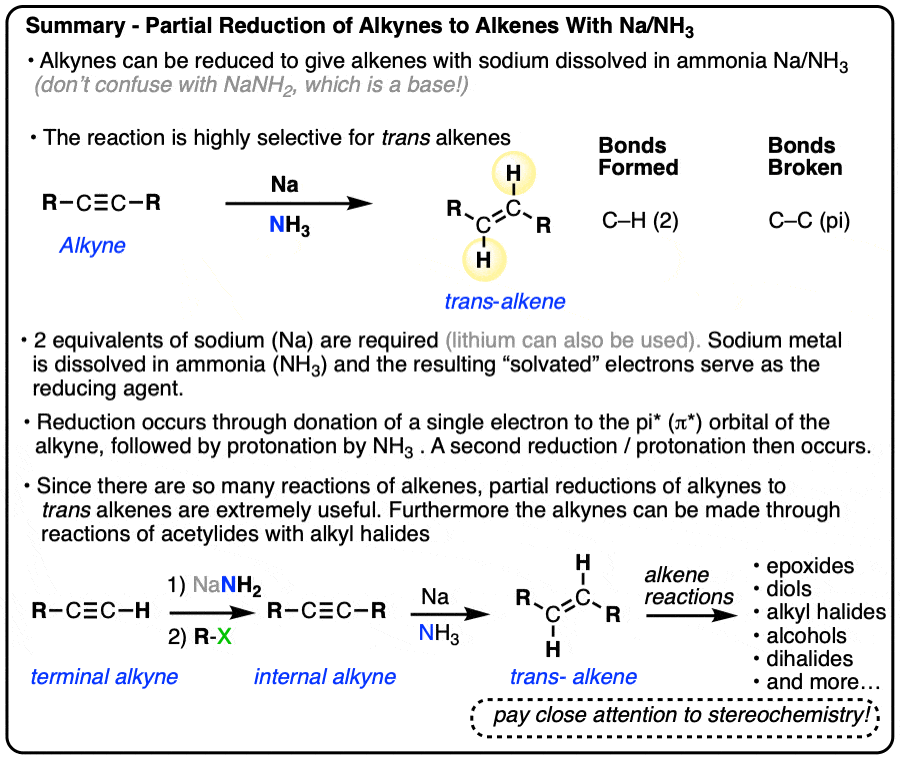
Table of Contents
1. Partial Reduction of Alkynes with Sodium in Ammonia (Na/NH3)
Previously we’ve seen that catalytic hydrogenation of alkynes with Pd/C and excess hydrogen gas (H2) gives alkanes (four C-H bonds formed, two C-C pi bonds broken). We’ve also seen that alkynes can be partially hydrogenated by using Lindlar’s catalyst, a hydrogenation catalyst where the Pd has intentionally been rendered less active (“poisoned”) through the addition of Pb (lead) salts. [See article – Partial Hydrogenation of Alkynes With Lindlar’s Catalyst]
When alkynes are hydrogenated with Lindlar’s catalyst, cis alkenes are formed preferentially:
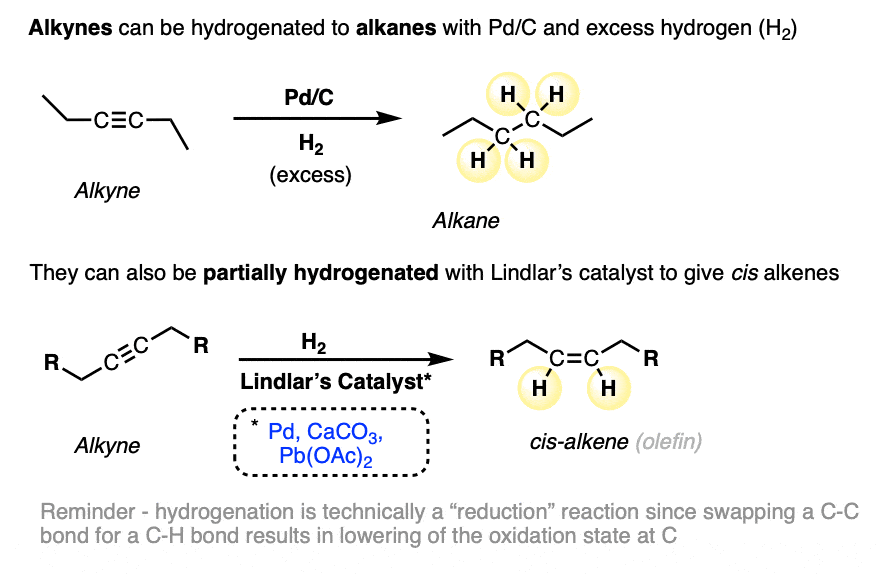
We call these hydrogenations “reductions” since each carbon is swapping a C-C bond for a C-H bond. In the oxidation state formalism, each C-H bond decreases the oxidation state of carbon by 1. For more, see article: How to calculate the oxidation state of a carbon).
A method for the conversion of alkynes into trans alkenes has also been developed. Instead of catalytic hydrogenation with palladium and H2, the alkyne is added into a solution of sodium (or lithium) metal dissolved in liquid ammonia (NH3).
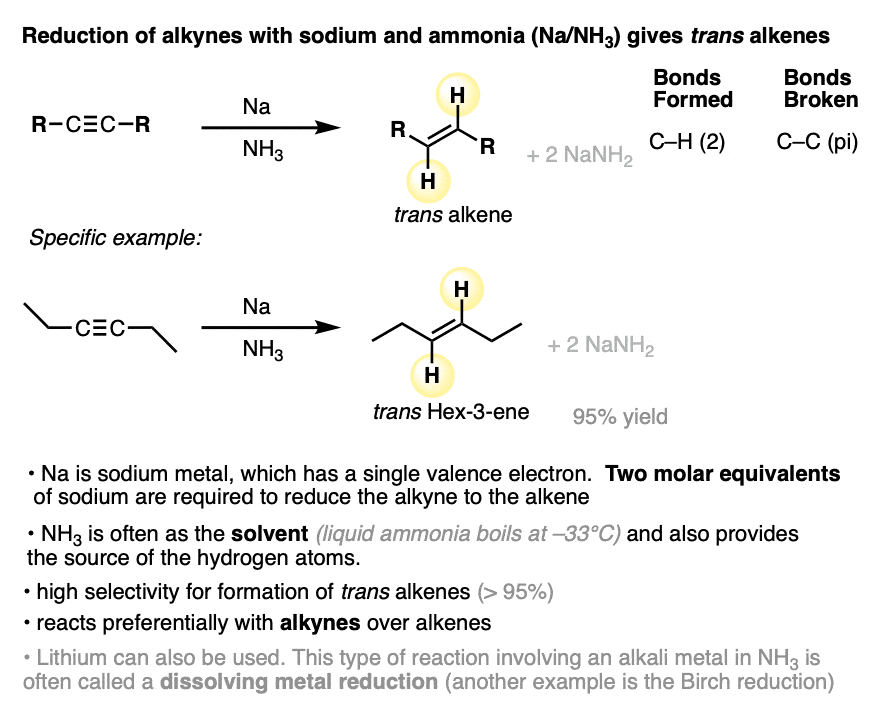
When sodium is dissolved in NH3, the loosely-held valence electron of Na dissociates from the metal, resulting in a solution of “solvated electrons”. These “free” electrons are the reducing agent in the conversion of alkynes to alkenes and are also responsible for the deep blue color of the solution.
This clip from @NileRed shows the addition of lithium, not sodium, but Na is very similar.

NH3 is a gas at room temperature. Using liquid NH3 as a solvent requires cooling it down to -33°C with an external cooling bath.
Alkynes are reduced to trans alkenes in good yields. Here are a few more examples.
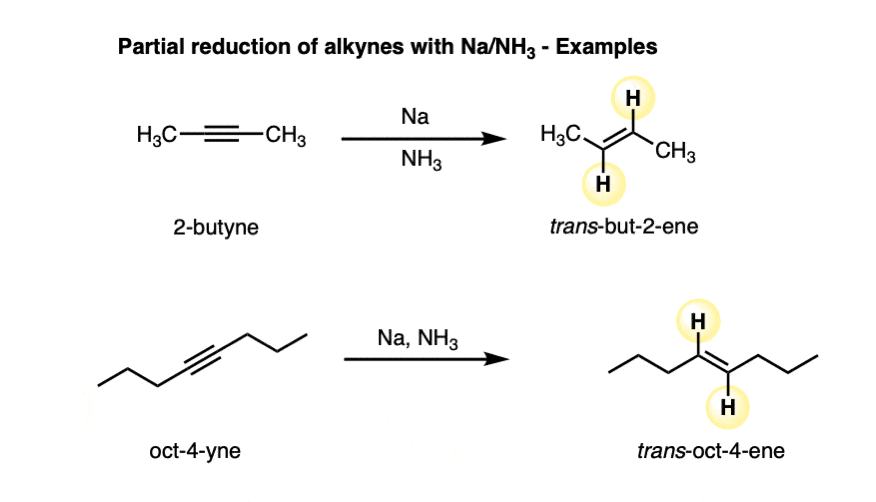
Since Na only has one valence electron, it’s important to add at least two molar equivalents of Na.
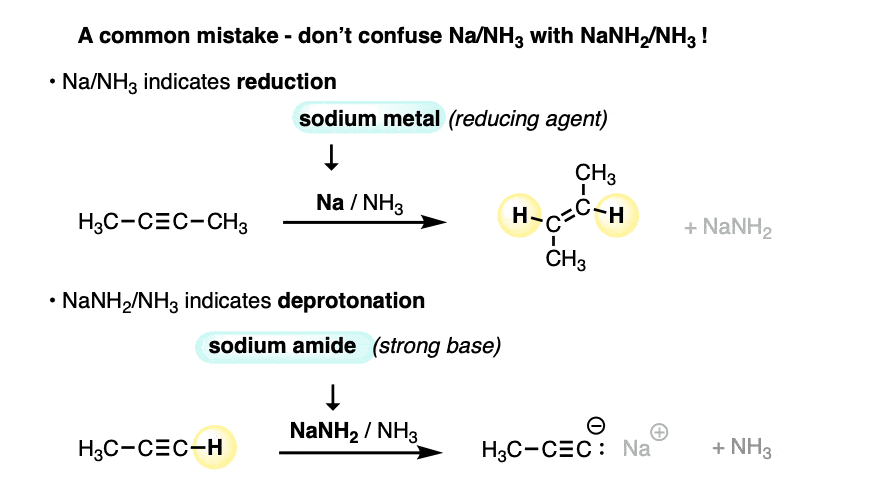
Make sure you don’t confuse Na/NH3 with NaNH2/NH3.
- NaNH2 is a strong base dissolved in its conjugate acid (ammonia). Useful for deprotonating terminal alkynes.
- Na is metallic sodium, a source of electrons (reducing agent) dissolved in ammonia.
Lithium can be used in place of sodium.
In practice, there are other ways of running this reaction that don’t involve liquid NH3, but we need not discuss them here. [Note 1].
2. The Consequences of Stereochemistry
It’s very useful to have a method for the partial reduction of alkynes to alkenes in your back pocket, since it allows us to employ the huge number of useful reactions of alkenes we have learned previously (See article – Reactions of Alkenes) .
Before we get too far ahead of ourselves, however, let’s just examine some consequences of the stereochemistry of this reaction.
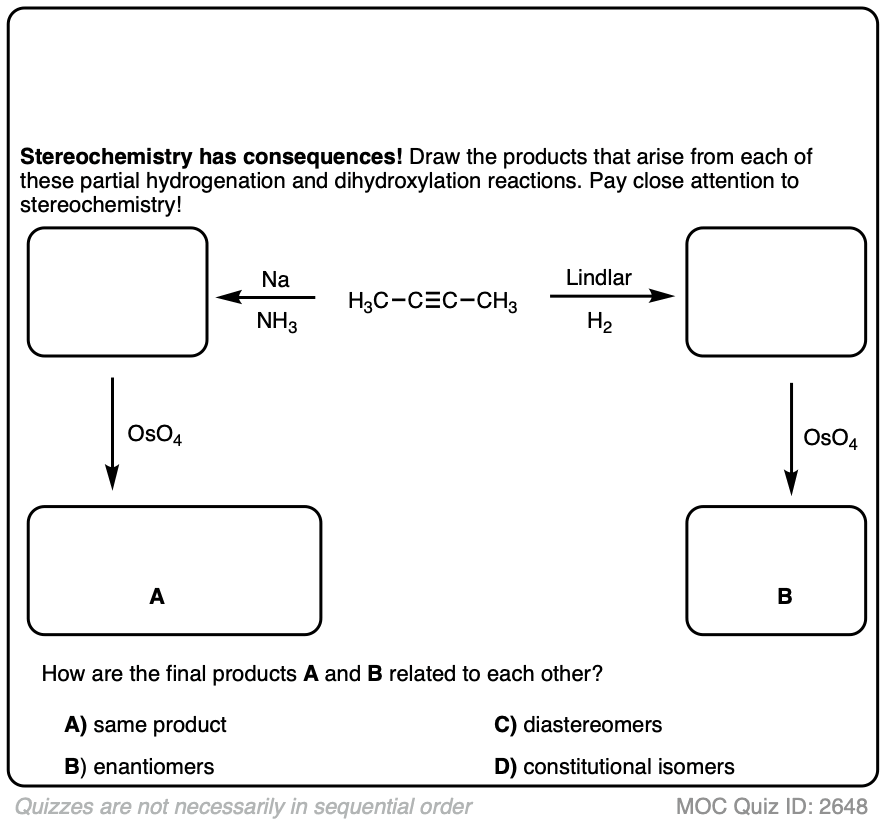
Starting with an alkyne, partial reduction via Lindlar/H2 will give you the cis alkene and reduction with Na/NH3 will give the trans alkene.
What happens when those respective alkenes are then subjected to a reaction known to be stereospecific, such as OsO4 for the dihydroxylation of alkenes?
How are those products related?
 Click to Flip
Click to Flip
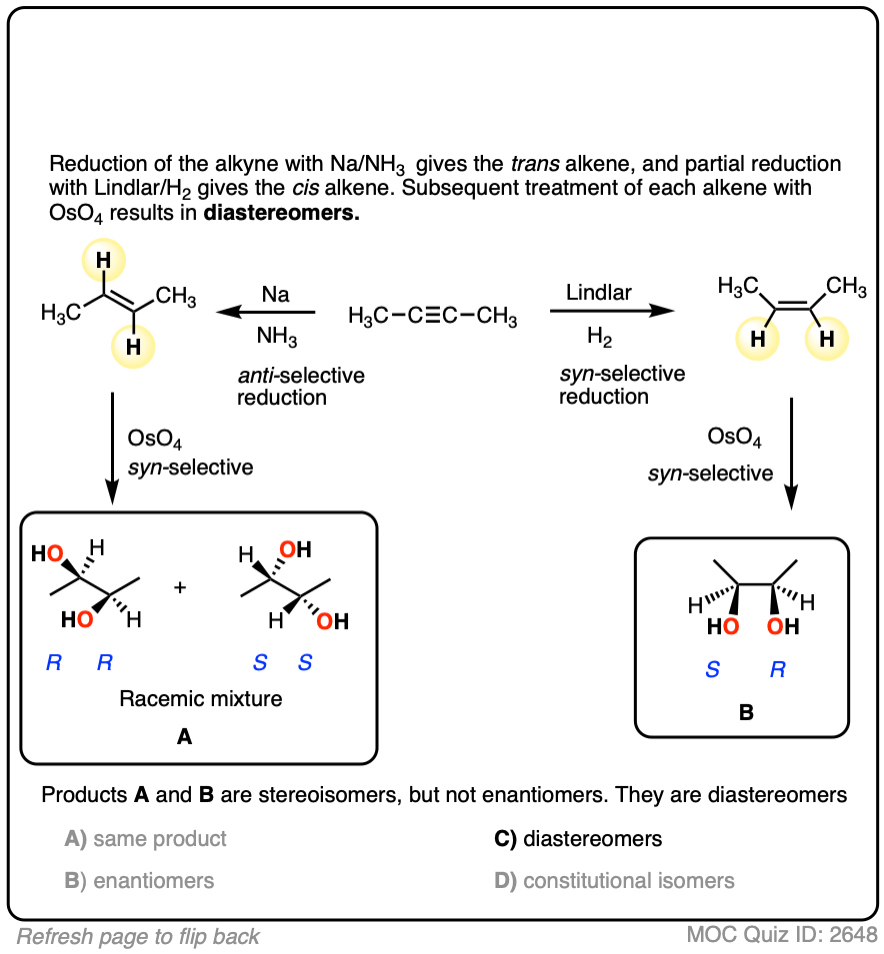
The result should be diastereomers.
You might rightfully ask whether or not it truly matters whether you use Na/NH3 or Lindlar in the first step. After all, don’t you still end up with a vicinal diol in the end? Those products look pretty much the same, don’t they?
They are not the same products! They have different melting points, different boiling points, different configurations in space. They will interact with chiral molecules (like proteins and enzymes!) in different ways. Not to get overly dramatic, but if a major pharmaecutical company sold a product to the public that had one stereocenter with the wrong configuration, the consequences could be death or disfigurement for thousands of its customers, with the attendant billions of dollars in lawsuits.
That’s why we care about stereochemistry. If we have the ability to control it, we should control it.
Here’s another example. Try drawing the product of these two reactions:
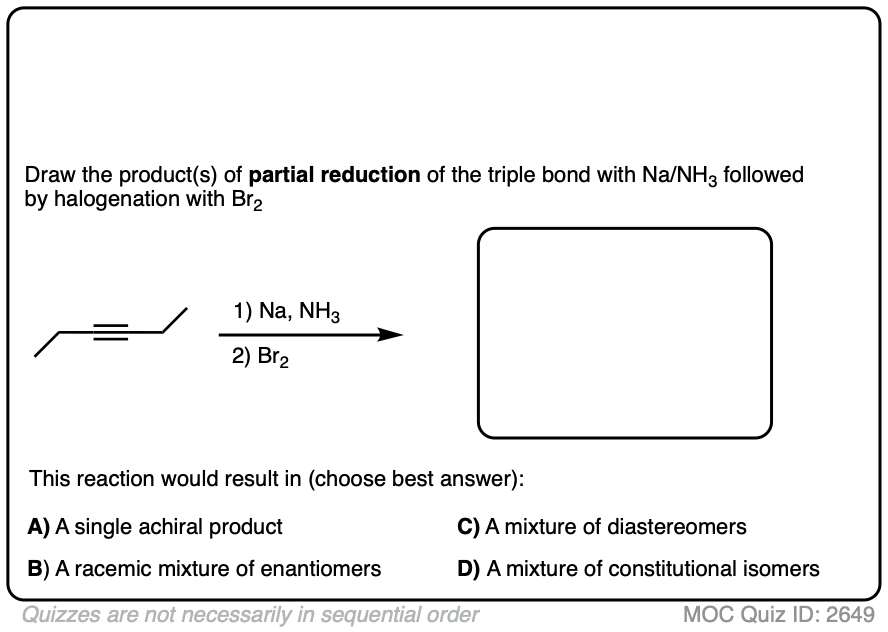 Click to Flip
Click to Flip
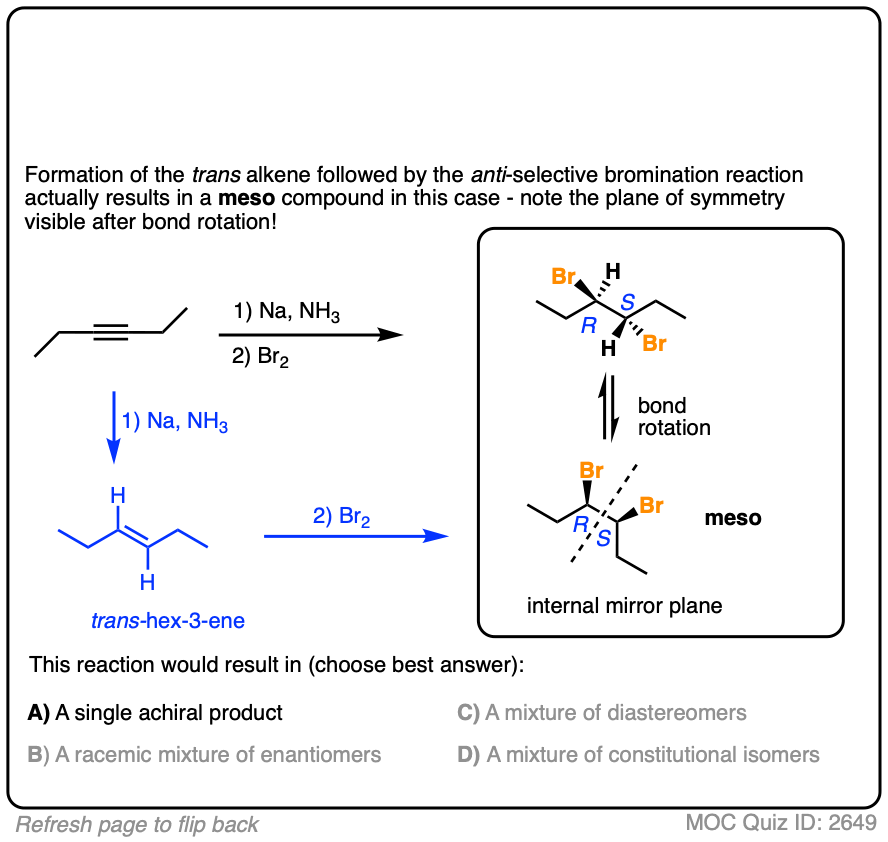
Final example. Draw the product!
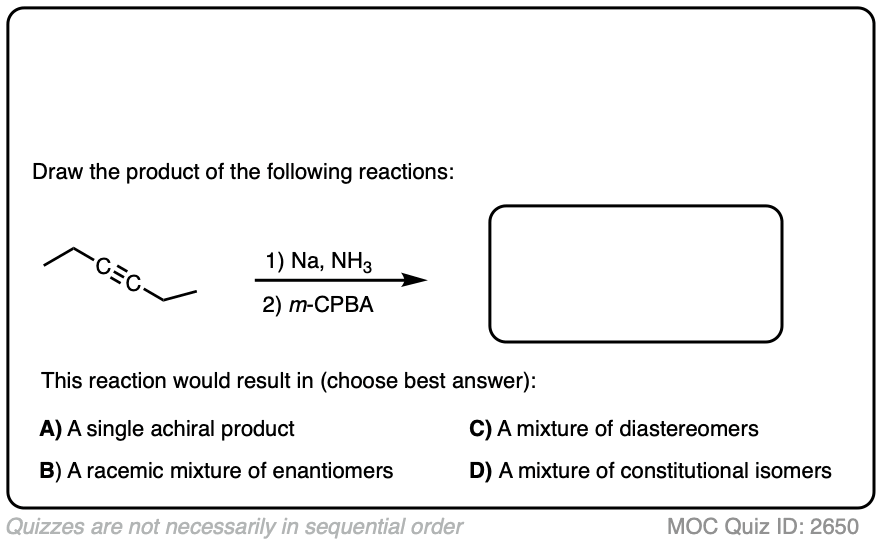 Click to Flip
Click to Flip
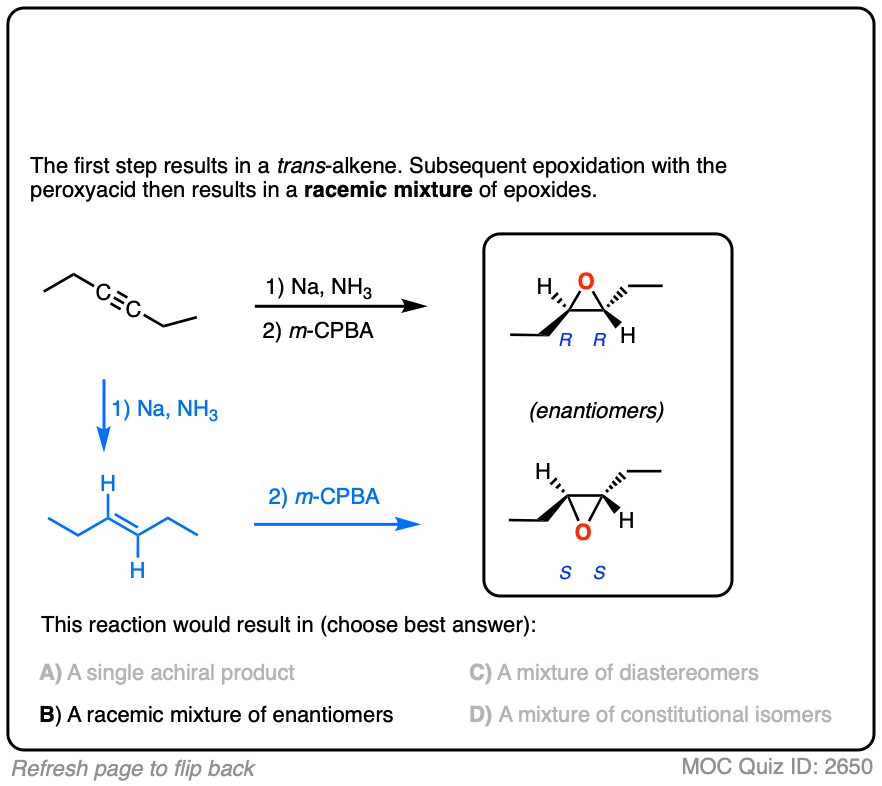
These are examples of questions that tie together topics in multiple chapters – stereochemistry, alkenes, and alkynes – and therefore make for excellent questions on final exams.
3. Mechanism For Reduction of Alkynes With Na/NH3
If Na is a source of electrons – a reducing agent – then where do those electrons go when the alkyne is added?
The electrons will go into the lowest-energy empty orbital available on the alkyne!
This can’t be a bonding orbital, however, because all the bonding orbitals are already occupied with electrons.
The lowest-energy unoccupied molecular orbital – often abbreviated LUMO – is actually the antibonding orbital of one of the pi bonds – in other words, a pi* orbital.
When the alkyne accepts an electron from solution, a pi-pi bond breaks, resulting in the formation of an intermediate which contains both an anion and a radical. Unsurprisingly, these types of species are called “radical anions”.
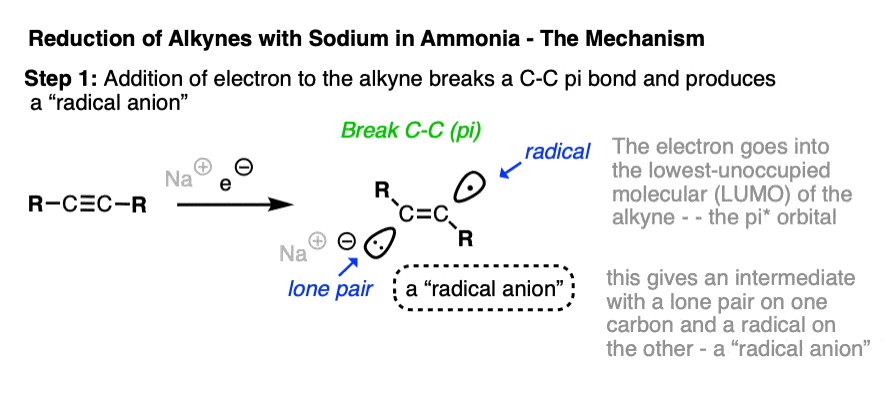
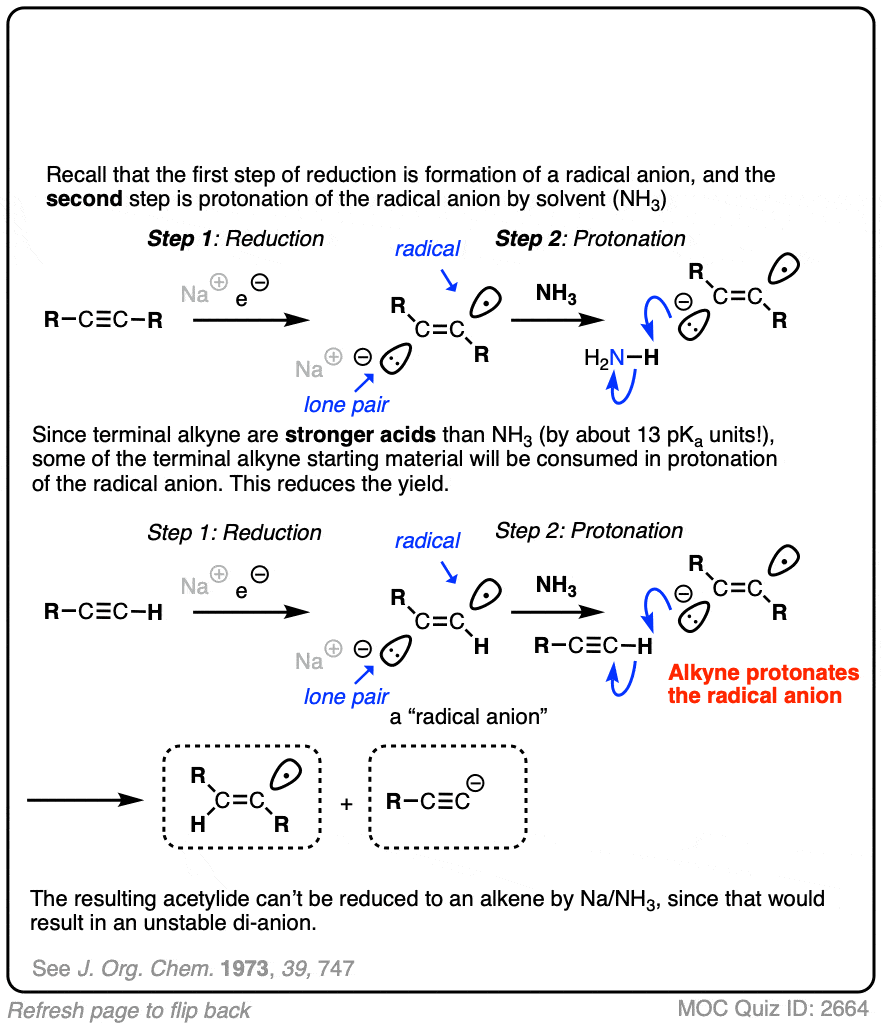
The next step is protonation of the anion by the strongest acid available, which in this case happens to be the solvent NH3.
Anions on sp2 hybridized carbons such as alkenes are extremely basic. Although we don’t generally think of NH3 as an acid (pKa 38), it is in fact a stronger acid than an alkene C-H (pKa about 42).
Since this acid-base reaction results in a weaker acid (NH3) and a weaker base (NaNH2 is a weaker base than the conjugate base of an alkene), the acid-base reaction is favorable overall. [See article – How To Use A pKa Table] [Note 2]
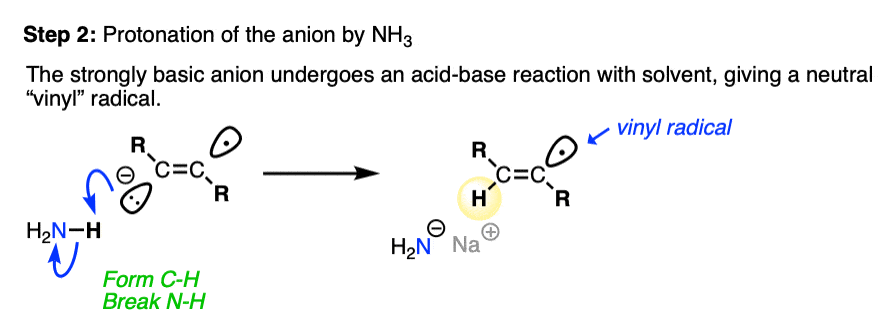
After protonation, we are left with a radical on the sp2 hybridized carbon of the alkene, a species known as a vinyl radical. [Note 3]
This vinyl radical rapidly accepts a second electron from solution, resulting in another anion.
It is generally thought that the preference for trans products is set at this stage. [Note 4]
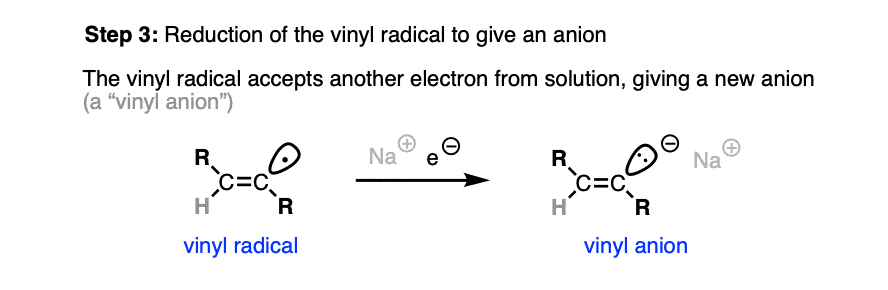
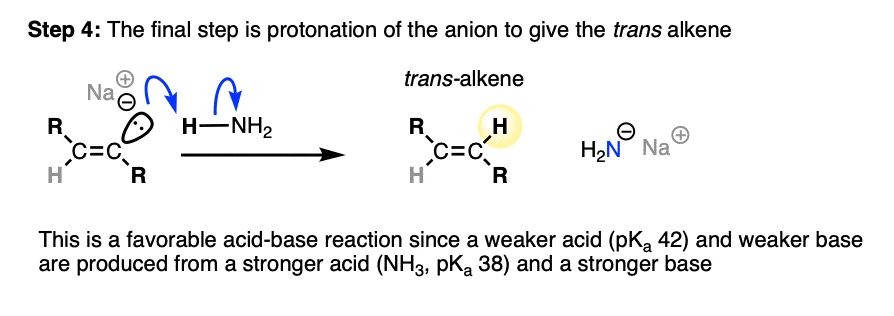
This anion is then protonated by solvent, giving us our alkene product.
4. Some Synthesis Problems Involving Na/NH3 Reduction
In a previous article, we saw that acetylides (the conjugate bases of alkynes) undergo SN2 reactions with alkyl halides to give substituted acetylenes. (See article – Substitution Reactions of Acetylides). This is an extremely important reaction for forming carbon-carbon bonds.
In the quizzes below, your job is to combine this reaction along with partial reduction and various reactions of alkenes to make several different products.
Sometimes doing a bond rotation will make the process of working backwards more clear. (See article – How To Do A Bond Rotation).
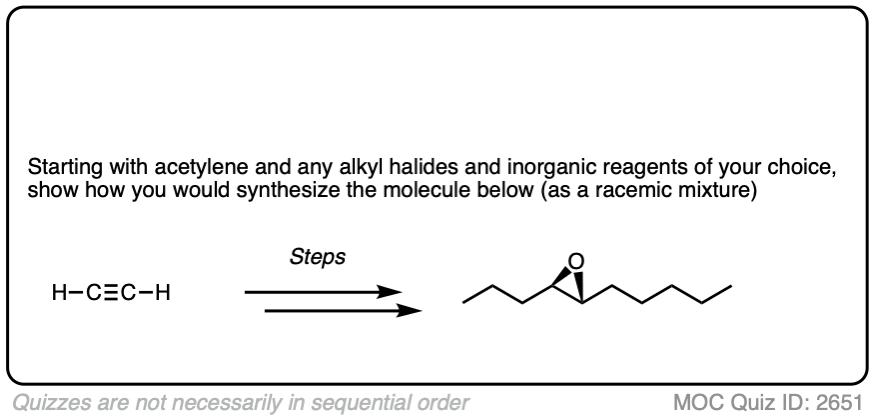
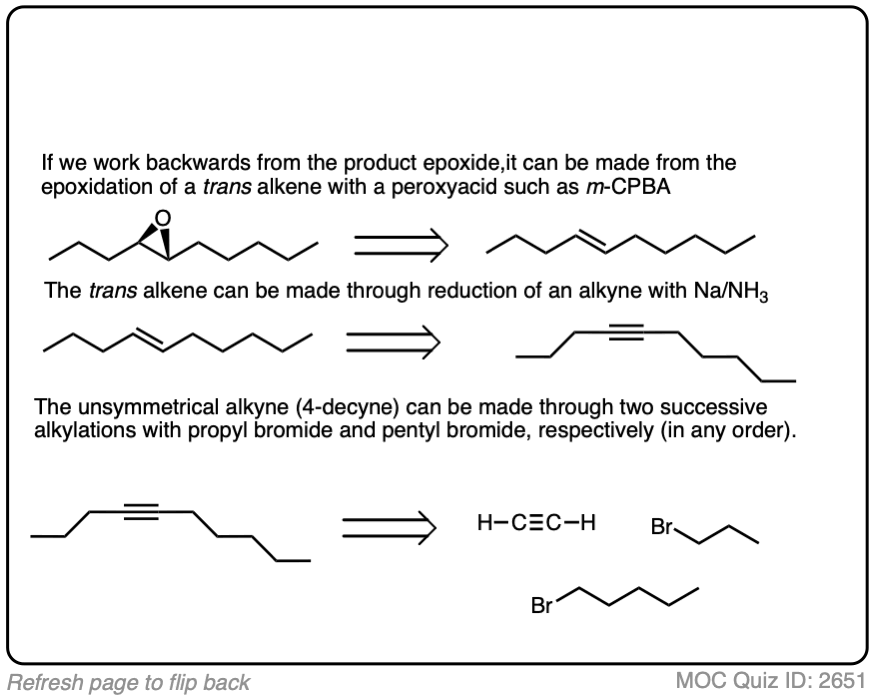
Work backwards from the product

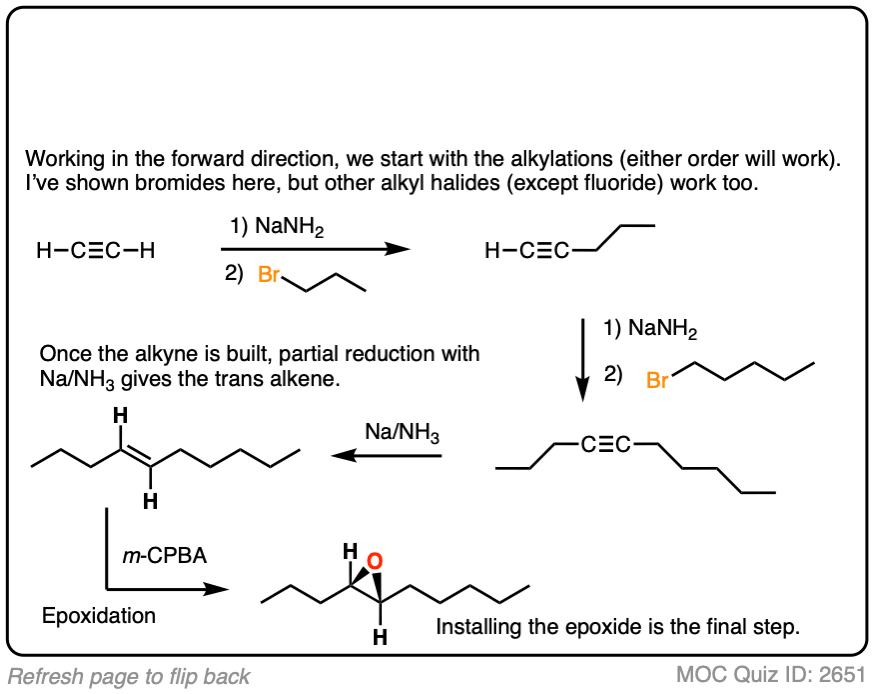
Final sequence

In this synthesis question, show how to build this vicinal diol starting from acetylene.
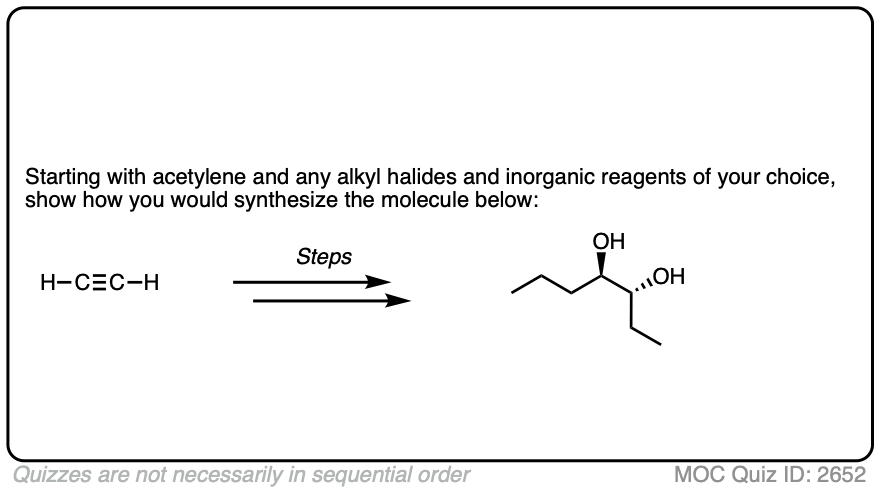
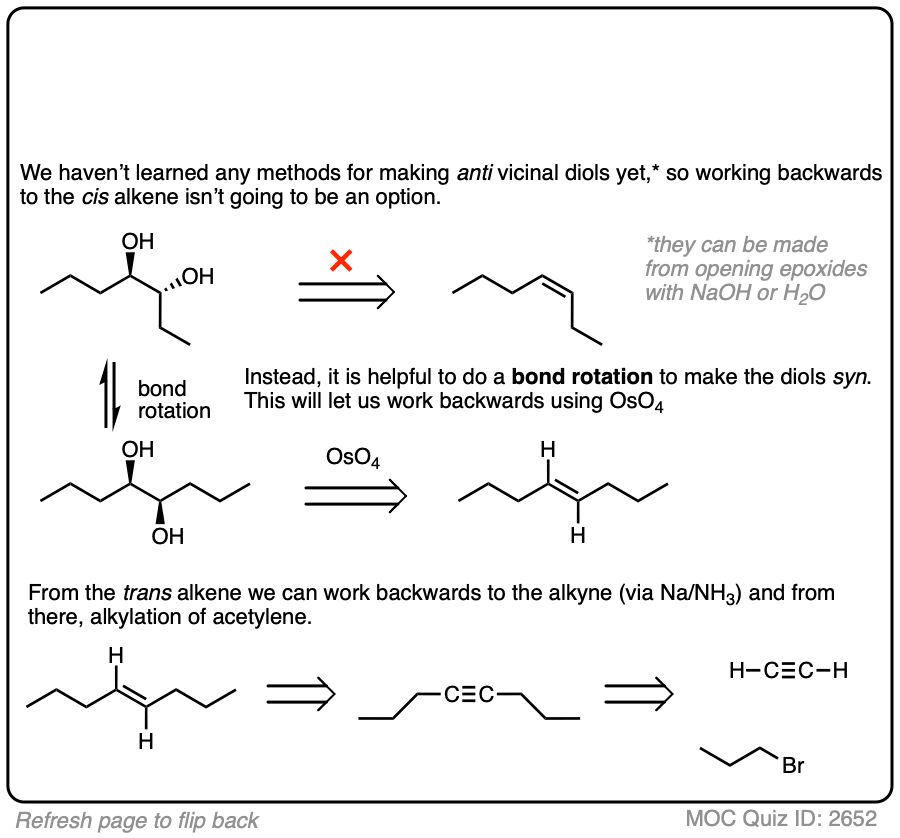
Work backwards from the product!

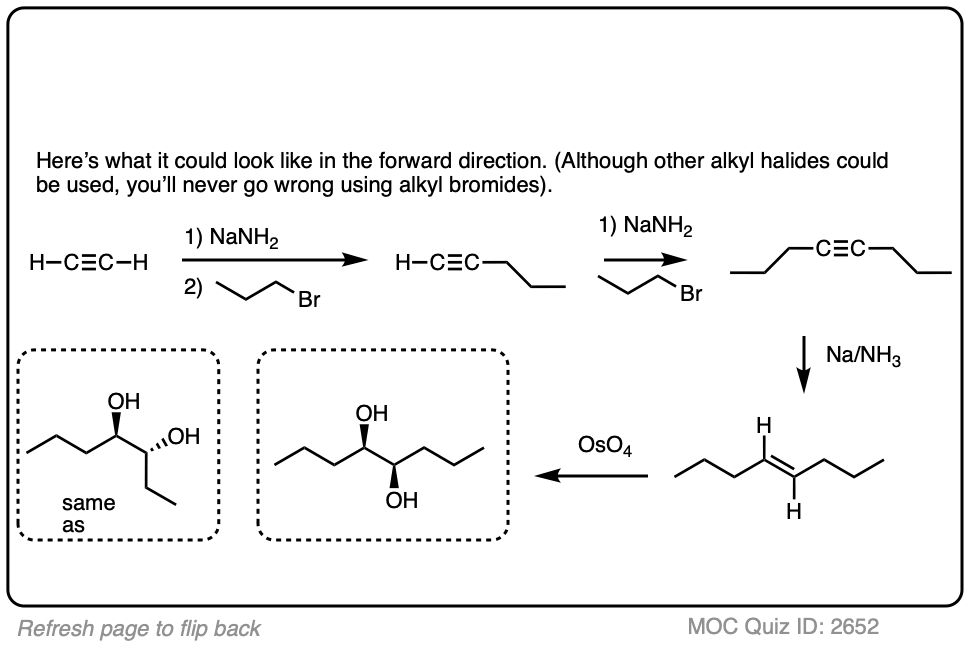
Final sequence

In the question below, show how you would make this vicinal dibromide.
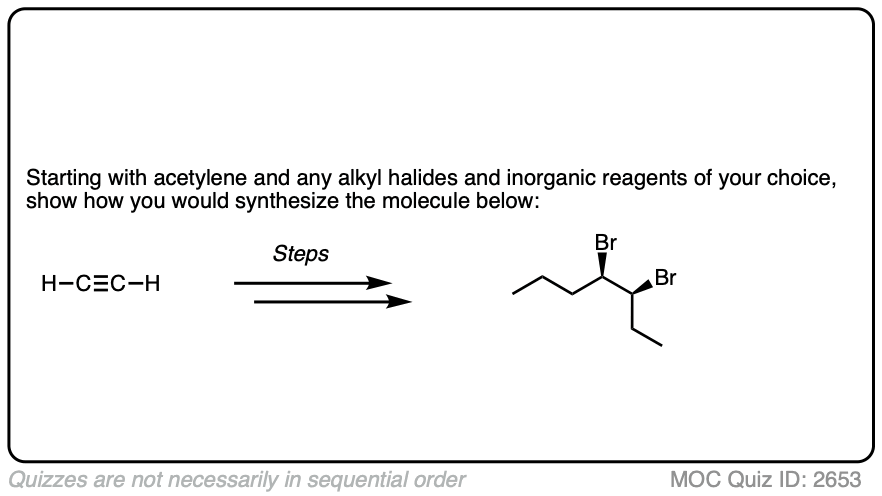
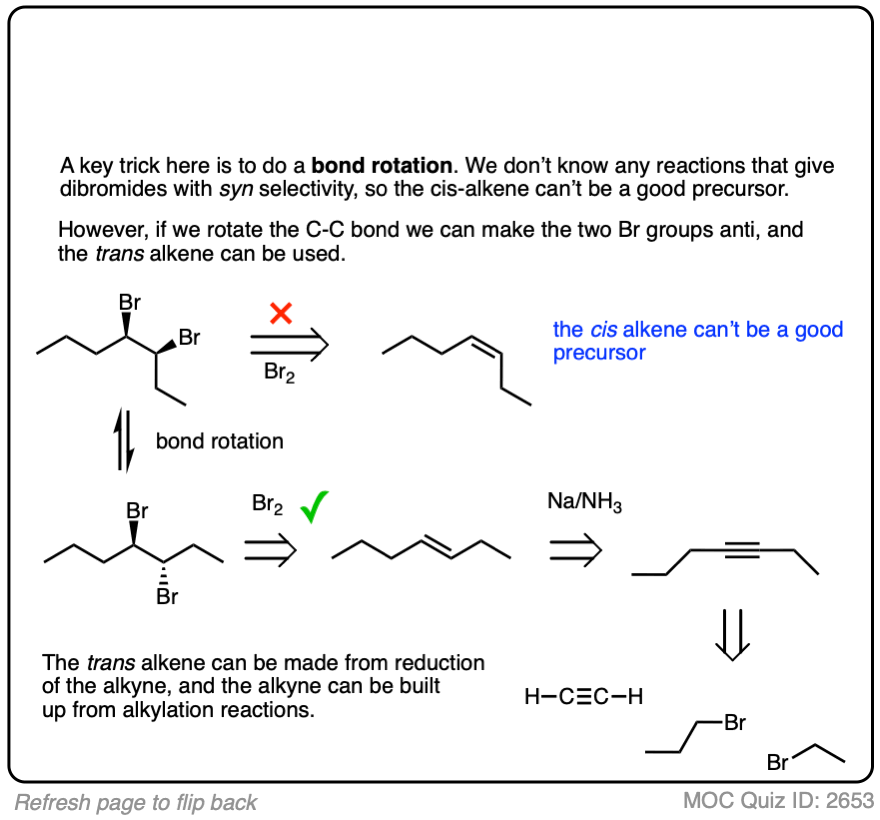
Work backwards from the final product

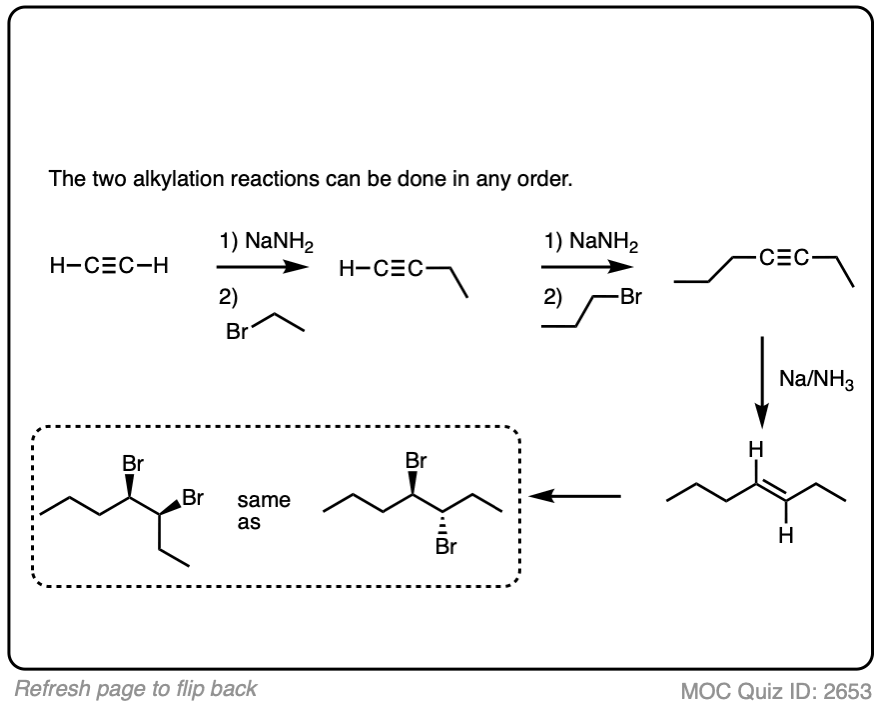
Put the reactions in proper sequence
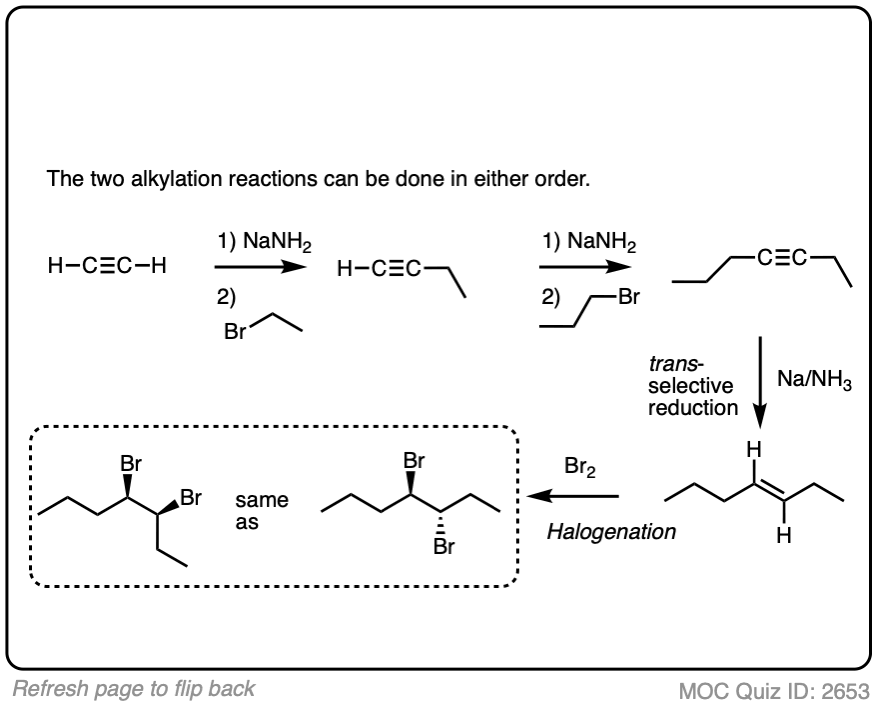
5. Summary
Alkynes are partially reduced to trans-alkenes by metallic sodium (Na) dissolved in liquid ammonia. The reaction is complimentary to the Lindlar reduction of alkynes, which gives cis products.
The resulting alkenes can then undergo all the reactions of alkenes we’ve learned in a previous chapter. It’s extremely important to pay attention to stereochemical details, both in the reduction itself and also in all the subsequent reactions of alkenes. Expect to see lots of exam-type questions that ask you to to synthesize various molecules from alkynes that involve Na/NH3 as a key step.
Notes
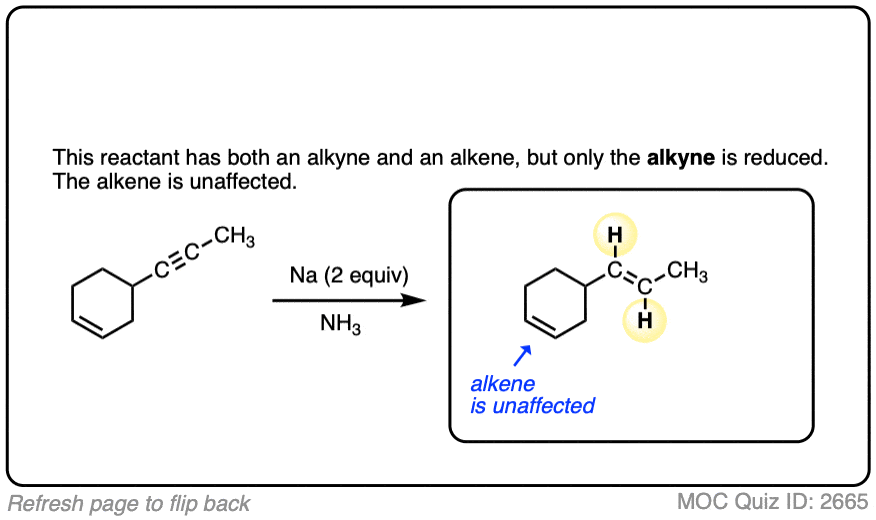
Note 1. Lithium can be used in place of sodium (Na). Also, solvents such as tetrahydrofuran (THF) can be used, so long as there is a source of protons – t-BuOH is a popular choice (similar to the Birch Reduction – see this article). The reaction is generally highly selective for reducing alkynes, but alkenes can be reduced if higher temperatures are employed.
For a pretty comprehensive study of different conditions for carrying out this reaction, see this article (House – JOC 1974 p. 747)
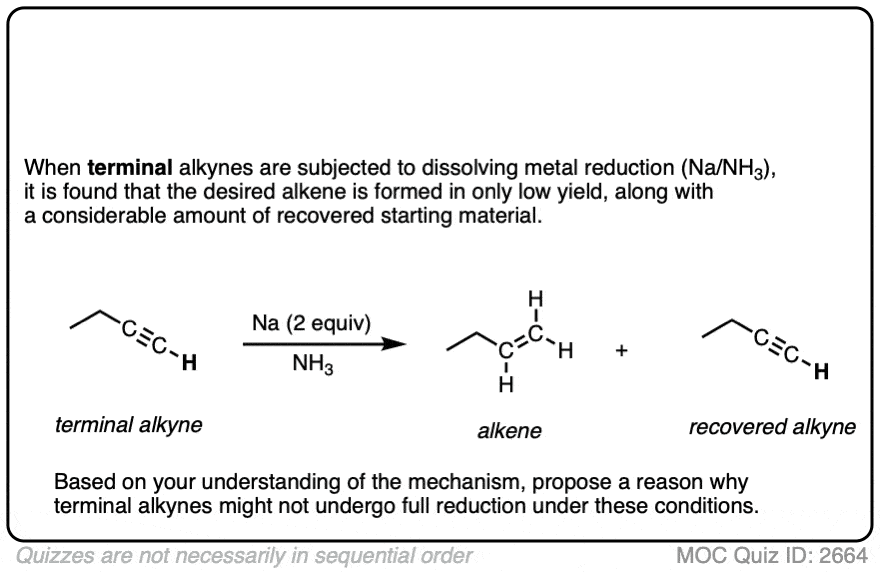
Note 2. Terminal alkynes are not very good substrates for these reductions. In one example, attempted reduction of a terminal alkyne resulted in a 31% yield, along with a considerable amount of recovered starting material. Can you figure out why? [Quiz 1]
Note 3. In the mechanism above, the vinyl radical is drawn trans. It’s been found that vinyl radicals can quite readily interconvert between their cis and trans forms, with a relatively low barrier to inversion (about 3 kcal/mol). Just for reference, this is roughly the energy barrier between staggered and eclipsed forms of ethane. So it’s extremely low, in other words!
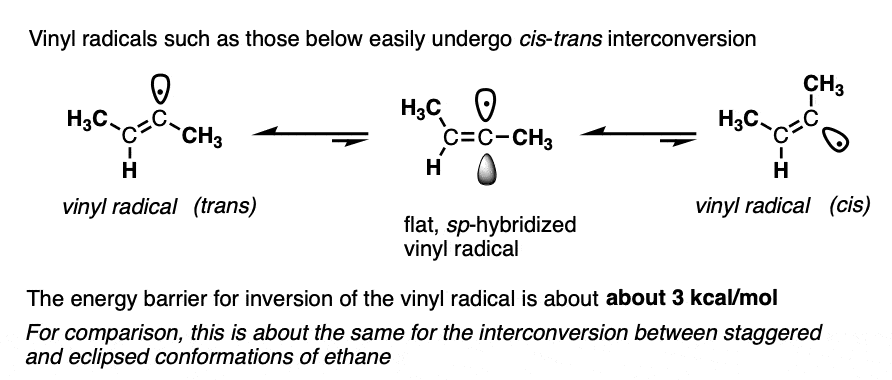
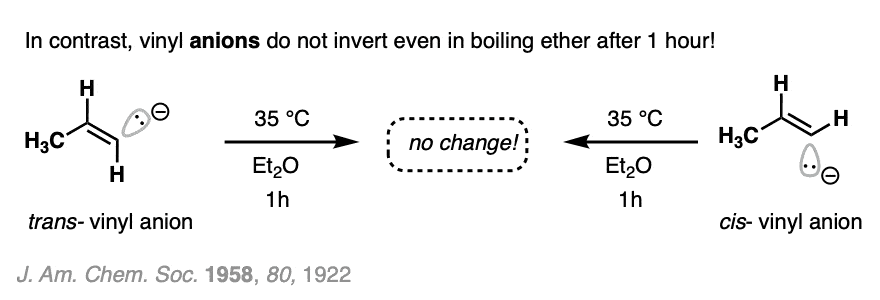
In contrast, vinyl anions are very stable. Once formed, they don’t generally lose their configurational geometry (27 kcal/mol). Incredibly, they can be heated in diethyl ether for an hour without losing their configuration.
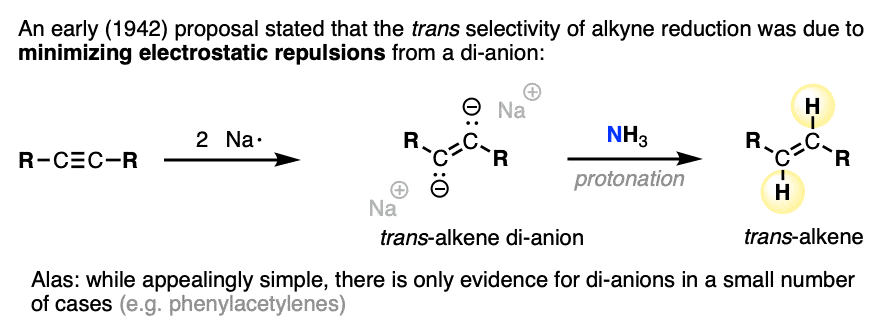
Note 4. The stereoselectivity for this reaction can be quite high (>95:5). Several studies have been made on the origins of the high stereoselectivity [Ref].
An early study proposed that the high trans selectivity was due to formation of a di-anion, which preferentially gave the trans product due to electrostatic lone-pair repulsion. It would be nice if this were true, since it is so intuitively simple. Alas, experiments and calculations indicate that dianions are generally too unstable to be formed under the reaction conditions, except in some special cases (e.g. diarylacetylenes).
The origin of the high trans selectivity has not been definitively established.
General consensus is that initial reduction of the alkyne preferentially forms a trans radical anion (exactly how favored this is, and to what extent it undergoes inversion to a cis radical anion, still needs to be studied more). After protonation, a vinyl radical is formed. It’s known that vinyl radicals have a low barrier to inversion (3 kcal/mol) so a big factor is the rate of the second reduction step relative to the rate of inversion of the vinyl radical. There can’t be complete equilibration between the cis and trans vinyl radicals, because they’ve each been made independently in the presence of Na, and the equilibrium ratio of trans:cis isomers after reduction is only about 80:20. [Ref] So the high selectivity (>95% in some conditions) must mean that reduction is fast, relative to equilibration, and must indicate some strong initial preference for formation of the trans radical anion. (indeed, if fewer equivalents of sodium are added, or if the temperature of the reaction is raised, the high selectivities erode back to about 80:20).
Quiz Yourself!
 Click to Flip
Click to Flip
(Advanced) References and Further Reading
- Reduction of Organic Compounds by Lithium in Low Molecular Weight Amines. 11. Stereochemistry. Chemical Reduction of an Isolated Nonterminal Double Bond
ROBERT A. BENKESER, GESE SCHROLI, AND DALE & I. SALVE
J. Am. Chem. Soc. 1955, 77, 12, 3378-3379
DOI: 10.1002/ja01617a066
Lithium and low-molecular weight amines (methylamine, ethylamine, isopropylamine) can also be used for dissolving metal reduction of alkynes. The advantage is that these reagents are easier to handle over sodium/ammonia. - The Preparation of Higher cis and trans Olefins
Kenneth N. Campbell and Lawrence T. Eby
Journal of the American Chemical Society 1941 63 (1), 216-219
DOI: 10.1021/ja01846a050
One of the first reports on the reduction of alkynes to trans alkenes with sodium and liquid NH3. Reductions of 5-decyne, 4-octyne, 3-octyne, and 3-hexyne proceed in good (>80%) yields. - Reactions involving electron transfer. V. Reduction on nonconjugated acetylenes
Herbert O. House and Edith F. Kinloch
The Journal of Organic Chemistry 1974 39 (6), 747-755
DOI: 10.1021/jo00920a002
Study of the product distribution of metal reduction of internal and terminal alkynes using Na in HMPA-THF. - Dissolving Metal Reduction of Acetylenes: A Computational Study
Robert Damrauer
The Journal of Organic Chemistry 2006 71 (24), 9165-9171
DOI: 10.1021/jo061583j
This is a computational investigation using DFT (density functional theory) which studies the stability of proposed intermediates in the dissolving metal reduction of acetylene, both in the gas phase and with explicit ammonia solvation.
“Findings include the probable bent nature of the radical anion species in ammonia, the likelihood that the trans stereochemistry of the reduction is determined at the vinyl anion stage, and the elimination of a dianion as a possible species that determines the stereochemical result.” - Configuration of vinyl radicals. The generation and trapping of each member of a configurationally isomeric pair of vinyl radicals
G. Dann. Sargent and M. Warren. Browne
Journal of the American Chemical Society 1967 89 (11), 2788-2790
DOI: 10.1021/ja00987a082
Some classic experiments here. The authors prepare pure E- and Z- 3-bromo-3-hexene, and subject them to one-electron reduction with lithium naphthalenide to make the vinyl radical, which is quickly trapped in solution. This lets them calculate the inversion barrier for vinyl radicals (about 3 kcal/mol). The (Z) vinyl radical rapidly equilibrates to give majority trans alkene. Once formed, pure (E) vinyl radical gives about 85:15 trans: cis alkenes. This is lower selectivity than what is typically seen in Na/NH3 reductions, so inversion of a vinyl radical would therefore seem unimportant in the mechanism for reduction of alkynes. - Reduction of alkyl- and arylacetylenes by an electrochemical method
Robert A. Benkeser and Cline A. Tincher
The Journal of Organic Chemistry 1968 33 (7), 2727-2730
DOI: 10.1021/jo01271a024 - Reduction of Organic Compounds by Lithium in Low Molecular Weight Amines. II. Stereochemistry. Chemical Reduction of an Isolated Non-terminal Double Bond
Robert A. Benkeser, Gene Schroll, and Dale M. Sauve
Journal of the American Chemical Society 1955 77 (12), 3378-3379
DOI: 10.1021/ja01617a066
Replacing liquid ammonia as solvent with other amines results in even more powerful reducing conditions. - Toxic Fluorine Compounds. XVIII.1 The Synthesis of the Toxic Principle of Dichapetalum toxicarium (18-Fluoro-cis-9-octadecenoic Acid) and Related ω-Fluoro Unsaturated Acids2,3
R. E. A. Dear and F. L. M. Pattison
Journal of the American Chemical Society 1963 85 (5), 622-626
DOI: 10.1021/ja00888a032
This paper contains a reduction with Na/NH3. - Many-Membered Carbon Rings. VI. Unsaturated Nine-membered Cyclic Hydrocarbons
A. T. Blomquist, Liang Huang Liu, and James C. Bohrer
Journal of the American Chemical Society 1952 74 (14), 3643-3647
DOI: 10.1021/ja01134a052
This article has an example of the reduction of cyclononyne to give trans-cyclononene.
This article is largely incorrect unfortunately.
The reaction of 2 eq. Na in ammonia with 3 eq. acetylene only yields 1 eq. of ethene, and 2 eq. of sodium acetylide. The sodium that reduces the acetylene to ethene turns into sodium amide, which deprotonates acetylene to form the acetylide.
For internal alkynes the reaction proceeds well with good yields.
The reaction works poorly for terminal alkynes, and even more poorly for acetylene. I mention the fact that the reaction works poorly for terminal alkynes in one of the quizzes.
Am I missing something? Because that doesn’t seem like much of a basis to call the article “largely incorrect”.
what happens in the reaction of alkynes with lithium aluminium hydride?
some sources say that LAH doesnt react with alkynes whereas some say partial hydrogenation takes place